Abstract
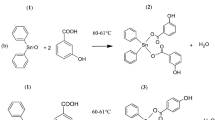
Nickel(II) complexes of 1H-tetrazol-5-acetic acid (H2L) and oligopyridines (1,10-phenanthroline /2,2’-bipyridine derivatives) have been synthesized and characterized by physicochemical methods (elemental and thermogravimetric analysis, powder X-ray diffraction, and IR spectroscopy). The behavior of the complexes in solution was studied by UV–Vis spectroscopy, conductometry, and mass spectrometry. The stability of the complexes over 48 h in aqueous solution and in phosphate-buffered saline was demonstrated using UV–Vis spectroscopy. These compounds were investigated for their cytotoxic and cytostatic activity against HepG2 (hepatocellular carcinoma), and Hep2 (larynx carcinoma) human cancer cell lines. Cytotoxicity was also studied on human non-cancerous cell line MRC-5 (lung fibroblast). All the compounds did not show cytotoxic activity against the tested cell lines in 1–50-µM concentration range. However, compounds showed a cytostatic effect against HepG2 and Hep2 cell lines. The most pronounced cytostatic properties were found for the complex [Ni(dmphen)2L]·2C2H5OH·2H2O (1). In addition, we report three new crystal structures: [Ni(phen)2L]·H2O, [Ni(dmbipy)2L]·2C2H5OH, and [Ni(dmphen)2L]·2C2H5OH·2H2O, where L2– behaves as a bidentate ligand which is coordinated to the Ni(II) ion via N,O atoms.



Similar content being viewed by others
References
Patil SA, Patil SA, Patil R, Keri RS, Budagumpi S, Balakrishna GR, Tacke M (2015) N-heterocyclic carbene metal complexes as bio-organometallic antimicrobial and anticancer drugs, Future. Med Chem 7:1305–1333. https://doi.org/10.4155/FMC.15.61
Naderi F, Orojloo M, Kamali S, Jannesar R, Amani S (2022) Synthesis, structural characterization, in vitro biological activity and computational quantum chemical studies of New Cobalt (II) Nickel (II) and Copper (II) Complexes Based on an Azo-Azomethine Ligand. Polycyclic Aromat Compd. https://doi.org/10.1080/10406638.2022.2049325
Cvek B, Milacic V, Taraba J, Dou QP (2008) Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J Med Chem 51:6256–6258. https://doi.org/10.1021/JM8007807
Dong X, Li Y, Li Z, Cui Y, Zhu H (2012) Synthesis, structures and urease inhibition studies of copper(II) and nickel(II) complexes with bidentate N, O-donor Schiff base ligands. J Inorg Biochem 108:22–29. https://doi.org/10.1016/J.JINORGBIO.2011.12.006
Satheesh CE, Raghavendra Kumar P, Sharma P, Lingaraju K, Palakshamurthy BS, Raja Naika H (2016) Synthesis, characterisation and antimicrobial activity of new palladium and nickel complexes containing Schiff bases. Inorganica Chim Acta. 442:1–9. https://doi.org/10.1016/J.ICA.2015.11.017
Benoist E, Gestin JF, Blanchard P, Jubault M, Quintard JP (1999) Spectroscopic and electrochemical studies of new copper(II) and nickel(II) N2S2 tetradentate complexes of potential interest to nuclear medicine. Transition Met Chem 24:42–48. https://doi.org/10.1023/A:1006939403074/METRICS
Millard JT (1999) Molecular probes of DNA structure, comprehensive natural products. Chemistry. https://doi.org/10.1016/B978-0-08-091283-7.00158-2
Kısa D, Korkmaz N, Taslimi P, Tuzun B, Tekin Ş, Karadag A, Şen F (2020) Bioactivity and molecular docking studies of some nickel complexes: New analogues for the treatment of Alzheimer, glaucoma and epileptic diseases. Bioorg Chem 101:104066. https://doi.org/10.1016/J.BIOORG.2020.104066
Sigel HSA (1996) Metal Ions In Biological Systems. CRC Press
Abu-Surrah A, Kettunen M (2006) Platinum group antitumor chemistry: design and development of new anticancer drugs complementary to cisplatin. Curr Med Chem 13:1337–1357. https://doi.org/10.2174/092986706776872970
Chohan ZH, Supuran CT, Scozzafava A (2008) Metalloantibiotics: synthesis and antibacterial activity of Cobalt(II), Copper(II), Nickel(II) and Zinc(II) Complexes of Kefzol. J Enzyme Inhib Med Chem 19:79–84. https://doi.org/10.1080/14756360310001624939
Chohan ZH, Arif M, Shafiq Z, Yaqub M, Supuran CT (2008) In vitro antibacterial, antifungal & cytotoxic activity of some isonicotinoylhydrazide Schiff’s bases and their cobalt (II), copper (II), nickel (II) and zinc (II) complexes. J Enzyme Inhib Med Chem 21:95–103. https://doi.org/10.1080/14756360500456806
Chohan ZH, Kausar S (1992) Biologically active complexes of nickel(II), copper(II) and zinc(II) with Schiff-base ligand derived from the reaction of 2-aminopyridine and pyrrol-2-carboxaldehyde–their synthesis and characterisation. Chem Pharm Bull 40:2555–2556. https://doi.org/10.1248/CPB.40.2555
Savir S, Wei ZJ, Liew JWK, Vythilingam I, Lim YAL, Saad HM, Sim KS, Tan KW (2020) Synthesis, cytotoxicity and antimalarial activities of thiosemicarbazones and their nickel (II) complexes. J Mol Struct 1211:128090. https://doi.org/10.1016/J.MOLSTRUC.2020.128090
Totta X, Papadopoulou AA, Hatzidimitriou AG, Papadopoulos A, Psomas G (2015) Synthesis, structure and biological activity of nickel(II) complexes with mefenamato and nitrogen-donor ligands. J Inorg Biochem 145:79–93. https://doi.org/10.1016/J.JINORGBIO.2015.01.009
Skyrianou KC, Perdih F, Papadopoulos AN, Turel I, Kessissoglou DP, Psomas G (2011) Nickel–quinolones interaction: Part 5—Biological evaluation of nickel(II) complexes with first-, second- and third-generation quinolones. J Inorg Biochem 105:1273–1285. https://doi.org/10.1016/J.JINORGBIO.2011.06.005
Sathyadevi P, Krishnamoorthy P, Jayanthi E, Butorac RR, Cowley AH, Dharmaraj N (2012) Studies on the effect of metal ions of hydrazone complexes on interaction with nucleic acids, bovine serum albumin and antioxidant properties. Inorganica Chim Acta 384:83–96. https://doi.org/10.1016/J.ICA.2011.11.033
Li Y, Yang ZY, Wu JC (2010) Synthesis, crystal structures, biological activities and fluorescence studies of transition metal complexes with 3-carbaldehyde chromone thiosemicarbazone. Eur J Med Chem 45:5692–5701. https://doi.org/10.1016/J.EJMECH.2010.09.025
Yu H, Zhang W, Yu Q, Huang FP, Bian HD, Liang H (2017) Ni(II) complexes with schiff base ligands: preparation, characterization, dna/protein interaction and cytotoxicity studies. Molecules 22:1772. https://doi.org/10.3390/MOLECULES22101772
Ray S, Asthana J, Tanski JM, Shaikh MM, Panda D, Ghosh P (2009) Design of nickel chelates of tetradentate N-heterocyclic carbenes with subdued cytotoxicity. J Organomet Chem 694:2328–2335. https://doi.org/10.1016/J.JORGANCHEM.2009.03.036
Buschini A, Pinelli S, Pellacani C, Giordani F, Ferrari MB, Bisceglie F, Giannetto M, Pelosi G, Tarasconi P (2009) Synthesis, characterization and deepening in the comprehension of the biological action mechanisms of a new nickel complex with antiproliferative activity. J Inorg Biochem 103:666–677. https://doi.org/10.1016/J.JINORGBIO.2008.12.016
Göktürk T, Topkaya C, Sakallı Çetin E, Güp R (2022) New trinuclear nickel(II) complexes as potential topoisomerase I/IIα inhibitors: in vitro DNA binding, cleavage and cytotoxicity against human cancer cell lines. Chem Pap 76:2093–2109. https://doi.org/10.1007/S11696-021-02005-Y
Varadaraji D, Suban SS, Ramasamy VR, Kubendiran K, Sankar J, Raguraman KG, Nalilu SK, Pati HN (2010) Synthesis and evaluation of a series of 1-substituted tetrazole derivatives as antimicrobial agents. Org Commun 3:45–56
Toney JH, Fitzgerald PMD, Grover-Sharma N, Olson SH, May WJ, Sundelof JG, Vanderwall DE, Cleary KA, Grant SK, Wu JK, Kozarich JW, Pompliano DL, Hammond GG (1998) Antibiotic sensitization using biphenyl tetrazoles as potent inhibitors of Bacteroides fragilis metallo-β-lactamase. Chem Biol 5:185–196. https://doi.org/10.1016/S1074-5521(98)90632-9
Myznikov LV, Hrabalek A, Koldobskii GI (2007) Drugs in the tetrazole series. (Review). Chem Heterocycl Compd (N Y). 43:1–9. https://doi.org/10.1007/S10593-007-0001-5
Lamie PF, Azmey AF (2019) Synthesis and biological evaluation of tetrazole derivatives as TNF-α, IL-6 and COX-2 inhibitors with antimicrobial activity: Computational analysis, molecular modeling study and region-specific cyclization using 2D NMR tools. Bioorg Chem. https://doi.org/10.1016/J.BIOORG.2019.103301
Hatamleh AA, Al Farraj D, Al-Saif SS, Chidambaram S, Radhakrishnan S, Akbar I (2020) Synthesis cytotoxic analysis, and molecular docking studies of tetrazole derivatives via N-mannich base condensation as potential antimicrobials. Drug Des Devel Ther. 14:4477–4492. https://doi.org/10.2147/DDDT.S270896
Labib MB, Fayez AM, E.S. EL-Nahass, M. Awadallah, P.A. Halim, (2020) Novel tetrazole-based selective COX-2 inhibitors Design, synthesis, anti-inflammatory activity, evaluation of PGE2, TNF-α, IL-6 and histopathological study. Bioorg Chem. https://doi.org/10.1016/J.BIOORG.2020.104308
Kabi AK, Sravani S, Gujjarappa R, Garg A, Vodnala N, Tyagi U, Kaldhi D, Velayutham R, Singh V, Gupta S, Malakar CC (2022) An overview on biological evaluation of tetrazole derivatives materials horizons. From Nature to Nanomaterials. https://doi.org/10.1007/978-981-16-8399-2_8/FIGURES/29
Ostrovskii VA, Popova EA, Trifonov RE (2017) Developments in tetrazole chemistry (2009–16). Adv Heterocycl Chem 123:1–62. https://doi.org/10.1016/BS.AIHCH.2016.12.003
Gao F, Xiao J, Huang G (2019) Current scenario of tetrazole hybrids for antibacterial activity. Eur J Med Chem 184:111744. https://doi.org/10.1016/J.EJMECH.2019.111744
Nami S, Aghebati-Maleki A, Morovati H, Aghebati-Maleki L (2019) Current antifungal drugs and immunotherapeutic approaches as promising strategies to treatment of fungal diseases. Biomed Pharmacother 110:857–868. https://doi.org/10.1016/J.BIOPHA.2018.12.009
Gonzalez-Lara MF, Sifuentes-Osornio J, Ostrosky-Zeichner L (2017) Drugs in clinical development for fungal infections. Drugs 77:1505–1518. https://doi.org/10.1007/S40265-017-0805-2/FIGURES/5
Wang SQ, Wang YF, Xu Z (2019) Tetrazole hybrids and their antifungal activities. Eur J Med Chem 170:225–234. https://doi.org/10.1016/J.EJMECH.2019.03.023
Bommagani S, Penthala NR, Balasubramaniam M, Kuravi S, Caldas-Lopes E, Guzman ML, Balusu R, Crooks PA (2019) A novel tetrazole analogue of resveratrol is a potent anticancer agent. Bioorg Med Chem Lett 29:172–178. https://doi.org/10.1016/J.BMCL.2018.12.006
Popova EA, Protas AV, Trifonov RE (2018) Tetrazole derivatives as promising anticancer agents. Anticancer Agents Med Chem. https://doi.org/10.2174/1871520617666170327143148
Zhang J, Wang S, Ba Y, Xu Z (2019) Tetrazole hybrids with potential anticancer activity. Eur J Med Chem 178:341–351. https://doi.org/10.1016/J.EJMECH.2019.05.071
Roh J, Karabanovich G, Vlčková H, Carazo A, Němeček J, Sychra P, Valášková L, Pavliš O, Stolaříková J, Klimešová V, Vávrová K, Pávek P, Hrabálek A (2017) Development of water-soluble 3,5-dinitrophenyl tetrazole and oxadiazole antitubercular agents. Bioorg Med Chem 25:5468–5476. https://doi.org/10.1016/J.BMC.2017.08.010
Gao C, Chang L, Xu Z, Yan XF, Ding C, Zhao F, Wu X, Feng LS (2019) Recent advances of tetrazole derivatives as potential anti-tubercular and anti-malarial agents. Eur J Med Chem 163:404–412. https://doi.org/10.1016/J.EJMECH.2018.12.001
Frija LMT, Rocha BGM, Kuznetsov ML, Cabral LIL, Cristiano MLS, Pombeiro AJL (2020) Well-defined nickel(II) tetrazole-saccharinate complex as homogeneous catalyst on the reduction of aldehydes: scope and reaction mechanism, pure and applied. Chemistry 92:151–166. https://doi.org/10.1515/PAC-2019-0220
Allab Y, Chikhi S, Zaater S, Brahimi M, Djebbar S (2020) Impact of the functionalized tetrazole ring on the electrochemical behavior and biological activities of novel nickel (II) complexes with a series of tetrazole derivatives. Inorganica Chim Acta 504:119436. https://doi.org/10.1016/J.ICA.2020.119436
Surendra Babu MS, Rao BU, Krishna V, Mustafa S, Rao GN (2017) Synthesis, characterization and DNA cleavage studies of isomeric pyridyl-tetrazole ligands and their Ni(II) and Zn(II) complexes. J Saudi Chem Soc 21:291–299. https://doi.org/10.1016/J.JSCS.2015.07.003
Ermakova EA, Golubeva JA, Smirnova KS, Klyushova LS, Eltsov IV, Zubenko AA, Fetisov LN, Svyatogorova AE, Lider EV (2023) Bioactive mixed-ligand zinc(II) complexes with 1H-tetrazole-5-acetic acid and oligopyridine derivatives. Polyhedron 230:116213. https://doi.org/10.1016/J.POLY.2022.116213
Ermakova EA, Golubeva YA, Smirnova KS, Klyushova LS, Berezin AS, Fetisov LN, Svyatogorova AE, Andros NO, Zubenko AA, Lider EV (2023) Cytotoxic mixed-ligand copper(II) complexes with 1H-tetrazole-5-acetic acid and oligopyridine derivatives. New J Chem 47:9472–9482. https://doi.org/10.1039/D3NJ00568B
APEX2, ver. 2.0; SAINT, ver. 8.18c; SADABS ver. 2.11; Bruker Advanced X-ray Solutions. Madison, Wisconsin, USA, 2000−2012, (n.d.).
Sheldrick GM (2015) SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr A 71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL, Acta Crystallogr C. Struct Chem 71:3–8. https://doi.org/10.1107/S2053229614024218
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: A complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Eremina JA, Ermakova EA, Smirnova KS, Klyushova LS, Berezin AS, Sukhikh TS, Zubenko AA, Fetisov LN, Kononenko KN, Lider EV (2021) Cu(II), Co(II), Mn(II) complexes with 5-phenyltetrazole and polypyridyl ligands: Synthesis, characterization and evaluation of the cytotoxicity and antimicrobial activity. Polyhedron 206:115352. https://doi.org/10.1016/J.POLY.2021.115352
Ermakova EA, Eremina JA, Smirnova KS, Klyushova LS, D.B. Kal’nyi, T.S. Sukhikh, A.A. Zubenko, L.N. Fetisov, K.N. Kononenko, E. V. Lider, (2021) Mixed-ligand manganese(II) complexes with 5-phenyltetrazole and polypyridine derivatives: Synthesis, crystal structures and biological activity. Results Chem. 3:100239. https://doi.org/10.1016/J.RECHEM.2021.100239
Eremina JA, Lider EV, Kuratieva NV, Samsonenko DG, Klyushova LS, D.G. Sheven’, R.E. Trifonov, V.A. Ostrovskii, (2021) Synthesis and crystal structures of cytotoxic mixed-ligand copper(II) complexes with alkyl tetrazole and polypyridine derivatives. Inorganica Chim Acta. 516:120169. https://doi.org/10.1016/J.ICA.2020.120169
Ali I, Wani WA, Saleem K (2013) Empirical formulae to molecular structures of metal complexes by molar conductance. Synth React Inorg Met-Org Nano-Met Chem 43:1162–1170. https://doi.org/10.1080/15533174.2012.756898
Meenongwa A, Brissos RF, Soikum C, Chaveerach P, Gamez P, Trongpanich Y, Chaveerach U (2016) Effects of N, N-heterocyclic ligands on the in vitro cytotoxicity and DNA interactions of copper(II) chloride complexes from amidino-O-methylurea ligands. New J Chem 40:586
Acknowledgements
The authors thank Anna P. Zubareva and Natalia N. Komardina for the elemental analysis, Aleksandra A. Shapovalova for IR spectroscopy, Anastasia O. Matveeva and Ilya V. Korolkov for the powder X-ray analysis data, and Vasiliy N. Yudin for providing the data collected in the XRD Facility of NIIC SB RAS. The research (Nikolaev Institute of Inorganic Chemistry SB RAS) was supported by the Ministry of Science and Higher Education of the Russian Federation, No. 121031700321-3. The work was performed using the equipment of the Center for Collective Use "Proteomic Analysis," supported by funding from the Ministry of Science and Higher Education of the Russian Federation (agreement No. 075-15-2021-691).
Funding
This work was supported by the Russian Science Foundation (Project no. 20–73-10207).
Author information
Authors and Affiliations
Contributions
E.A.E. wrote the main manuscript text, investigation, visualization Yu.A.G.: investigation, review and editing of the manuscript K.S.S.: investigation, visualization, wrote description of crystal structures L.S.K.: investigation in vitro cytotoxicity study, visualization D.G.Sh.: investigation by mass spectrometry E.V.L.: review and editing of the manuscript, conceptualization, supervision
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ermakova, E.A., Golubeva, Y.A., Smirnova, K.S. et al. Synthesis, structural characterization, and cytotoxicity of nickel(II) complexes with 1H-tetrazole-5-acetic acid and oligopyridines. Transit Met Chem (2024). https://doi.org/10.1007/s11243-024-00573-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11243-024-00573-y