Abstract
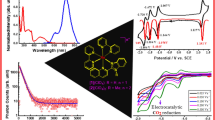
The six-coordinate ruthenium(II) porphyrin complexes (OEP)Ru(CO)(Q), (OEP = 2,3,7,8,12,13,17,18-octaethylporphyrinato dianion; Q = quinoline, Qnl (2); quinine, QN (3)) have been prepared from (OEP)Ru(CO) (1) and characterized by MS, IR, UV–visible and 1H NMR spectroscopy. The X-ray crystal structure of 2 has been determined, which reveals quinoline coordination to Ru through the nitrogen atom. In the crystal packing of 2, the two Qnl groups of adjacent porphyrins are positioned relatively parallel to each other at a close distance of 3.30 Å, implying a relatively strong π-π interaction. The X-ray crystal structure of 1 was obtained, which revealed coordination of the water to the ruthenium center. By comparing the spectroscopic data for 1, 2 and 3, it was determined that the site of binding of QN to Ru is likely through the nitrogen atom of the quinoline moiety. The redox behavior of the complexes at a Pt working electrode studied in a CH2Cl2 solution with NBu4PF6 as support electrolyte by cyclic voltammetry revealed oxidations that are porphyrin-centered.
Graphical abstract









Similar content being viewed by others
References
Afzal O, Kumar S, Haider MR, Ali MR, Kumar R, Jaggi M, Bawa S (2015) A review on anticancer potential of bioactive heterocycle quinoline. Eur J Med Chem 97:871–910. https://doi.org/10.1016/j.ejmech.2014.07.044
Mukherjee S, Pal M (2013) Medicinal chemistry of quinolines as emerging anti-inflammatory agents: an overview. Curr Med Chem 20(35):4386–4410. https://doi.org/10.2174/09298673113209990170
Matada BS, Pattanashettar R, Yernale NG (2021) A comprehensive review on the biological interest of quinoline and its derivatives. Bioorgan Med Chem 32:115973. https://doi.org/10.1016/j.bmc.2020.115973
Gorka AP, de Dios A, Roepe PD (2013) Quinoline drug-heme interactions and implications for antimalarial cytostatic versus cytocidal activities. J Med Chem 56(13):5231–5246. https://doi.org/10.1021/jm400282d
Weissbuch I, Leiserowitz L (2008) Interplay between malaria, crystalline Hemozoin formation, and antimalarial drug action and design. Chem Rev 108(11):4899–4914. https://doi.org/10.1021/cr078274t
Kapishnikov S, Hempelmann E, Elbaum M, Als-Nielsen J, Leiserowitz L (2021) Malaria pigment crystals: the Achilles′ Heel of the Malaria parasite. ChemMedChem 16(10):1515–1532. https://doi.org/10.1002/cmdc.202000895
de Villiers KA, Egan TJ (2021) Heme detoxification in the malaria parasite: a target for antimalarial drug development. Accounts Chem Res 54(11):2649–2659. https://doi.org/10.1021/acs.accounts.1c00154
de Dios AC, Tycko R, Ursos LMB, Roepe PD (2003) NMR studies of chloroquine−ferriprotoporphyrin IX complex. J Phys Chem A 107(30):5821–5825. https://doi.org/10.1021/jp0342982
Alumasa JN, Gorka AP, Casabianca LB, Comstock E, de Dios AC, Roepe PD (2011) The hydroxyl functionality and a rigid proximal N are required for forming a novel non-covalent quinine-heme complex. J Inorg Biochem 105(3):467–475. https://doi.org/10.1016/j.jinorgbio.2010.08.011
Constantinidis I, Satterlee JD (1988) UV-visible and carbon NMR studies of quinine binding to urohemin I chloride and uroporphyrin I in aqueous solution. J Am Chem Soc 110(3):927–932. https://doi.org/10.1021/ja00211a037
de Villiers KA, Gildenhuys J, le Roex T (2012) Iron(III) protoporphyrin IX complexes of the antimalarial cinchona alkaloids quinine and quinidine. ACS Chem Biol 7(4):666–671. https://doi.org/10.1021/cb200528z
Gildenhuys J, Sammy CJ, Müller R, Streltsov VA, le Roex T, Kuter D, de Villiers KA (2015) Alkoxide coordination of iron(iii) protoporphyrin IX by antimalarial quinoline methanols: a key interaction observed in the solid-state and solution. Dalton Trans 44(38):16767–16777. https://doi.org/10.1039/C5DT02671G
Kuter D, Streltsov V, Davydova N, Venter GA, Naidoo KJ, Egan TJ (2016) Solution structures of chloroquine–ferriheme complexes modeled using MD simulation and investigated by EXAFS spectroscopy. J Inorg Biochem 154:114–125. https://doi.org/10.1016/j.jinorgbio.2015.06.010
Buller R, Peterson ML, Almarsson Ö, Leiserowitz L (2002) Quinoline binding site on malaria pigment crystal: a rational pathway for antimalaria drug design. Cryst Growth Des 2(6):553–562. https://doi.org/10.1021/cg025550i
Sanders JKM, Bampos N, Clyde-Watson Z, Darling SL, Hawley JC, Kim H-J, Mak CCM, Webb SJ (2000) In: Kadish K, Smith KM, Guillard, R (eds) The Porphyrin Handbook, Vol. 3 Academic Press, New York, p. 1–48. And references therein.
Buchler JW, Dreher C, Künzel FM (1995) Synthesis and coordination chemistry of noble metal porphyrins. Metal Complexes with Tetrapyrrole Ligands III. Berlin, Heidelberg: Springer Berlin Heidelberg, 1–69. And references therein.
Awasabisah D, Xu N, Sharmah Gautam KP, Powell DR, Shaw MJ, Richter-Addo GB (2013) Stable ruthenium nitrosyl porphyrins with axial O-bonded ligands; preparation and redox behavior. Dalton Trans 42(24):8537–8540. https://doi.org/10.1039/C3DT33109A
Awasabisah D, Xu N, Gautam KPS, Powell DR, Shaw MJ, Richter-Addo GB (2016) Preparation, characterization, electrochemistry, and infrared spectroelectrochemistry of ruthenium nitrosyl porphyrins containing η1-o-bonded axial carboxylates. Eur J Inorg Chem 4:509–518. https://doi.org/10.1002/ejic.201501115
Cheng L, Richter-Addo GB (2000) Binding and activation of nitric oxide by metalloporphyrins and heme. In: Kadish KM, Smith KM, Guilard R (eds) The Porphyrin Handbook, vol 4. Academic Press, New York, pp 219–291 (And references therein)
Barley M, Dolphin D, James BR, Kirmaier C, Holten D (1984) Picosecond flash photolysis of carbonyl complexes of ruthenium(II) porphyrin pi cation radicals. J Am Chem Soc 106(14):3937–43
Bruker Nano, Inc. Data Collection: APEX3. Madison, Wisconsin: Bruker Nano Inc. 2018.
Bruker Nano, Inc. Data Reduction SAINT. Madison, Wisconsin: Bruker Nano Inc; 2016.
Rillema DP, Nagle JK, Barringer LF, Meyer TJ (1981) Redox properties of metalloporphyrin excited states, lifetimes, and related properties of a series of para-substituted tetraphenylporphine carbonyl complexes of ruthenium(II). J Am Chem Soc 103(1):56–62. https://doi.org/10.1021/ja00391a013
Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D (2015) Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J Appl Crystallogr 48(1):3–10. https://doi.org/10.1107/S1600576714022985
Sheldrick GM (2015) SHELXT– Integrated space-group and crystal-structure determination. Acta Crystallogr A 71(1):3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71(1):3–8. https://doi.org/10.1107/S2053229614024218
Macrae CF, Sovago I, Cottrell SJ, Galek PTA, McCabe P, Pidcock E, Platings M, Shields GP, Stevens JS, Towler M, Wood PA (2020) Mercury 4 0: from visualization to analysis, design and prediction. J Appl Crystallogr 53(1):226–35. https://doi.org/10.1107/S1600576719014092
Eaton GR, Eaton SS (1997) Reversible carbon monoxide binding by ruthenium carbonyl porphyrins. J Am Chem Soc 97(1):235–236. https://doi.org/10.1021/ja00834a066
Chow BC, Cohen IA (1971) Derivatives of tetraphenylporphineruthenium (II). Bioinorg Chem 1(1):57–63. https://doi.org/10.1016/S0006-3061(71)80001-7
Abraham RJ, Medforth CJ (1988) The NMR spectra of the porphyrins 36—Ring currents in octaethylporphyrin, meso-tetraphenylporphyrin and phthalocyanine complexes. Magn Reson Chem 26(9):803–12
Gouterman M (1978) Optical spectra and electronic structure of porphyrins and related rings. In: Dolphin D (ed) The porphyrins. Academic Press, pp 1–165
Sangster AW, Stuart KL (1965) Ultraviolet spectra of alkaloids. Chem Rev 65(1):69–130. https://doi.org/10.1021/cr60233a003
Antipas A, Buchler JW, Gouterman M, Smith PD (1978) Synthesis and optical and electronic properties of some ruthenium and osmium octaethylporphyrins. J Am Chem Soc 100(10):3015–3024. https://doi.org/10.1021/ja00478a013
Headen TF, Howard CA, Skipper NT, Wilkinson MA, Bowron DT, Soper AK (2010) Structure of π−π interactions in aromatic liquids. J Am Chem Soc 132(16):5735–5742. https://doi.org/10.1021/ja909084e
Salzmann R, McMahon MT, Godbout N, Sanders LK, Wojdelski M, Oldfield E (1999) Solid-State NMR, crystallographic and density functional theory investigation of Fe−CO and Fe−CO analogue metalloporphyrins and metalloproteins. J Am Chem Soc 121(16):3818–3828. https://doi.org/10.1021/ja9832818
Shirokova VV, Tyurin VS, Stanetskaya NM, Sokolova MN, Shkirdova AO, Zamilatskov IA (2019) The structure of complexes of carbonylruthenium(II) with octaethylporphyrin. Prot Met Phys Chem S 55(6):1104–1112. https://doi.org/10.1134/S2070205119060285
Hopf FR, O’Brien TP, Scheidt WR, Whitten DG (1975) Structure and reactivity of ruthenium(II) porphyrin complexes. Photochemical ligand ejection and formation of ruthenium porphyrin dimers. J Am Chem Soc 97(2):277–81
Rebouças JS, Cheu ELS, Ware CJ, James BR, Skov KA (2008) Synthetic and mechanistic aspects of a new method for ruthenium-metalation of porphyrins and schiff-bases. Inorg Chem 47(17):7894–7907. https://doi.org/10.1021/ic800616q
Collman JP, Barnes CE, Brothers PJ, Collins TJ, Ozawa T, Gallucci JC, Ibers JA (1984) Oxidation of ruthenium(II) and ruthenium(III) porphyrins Crystal structures of µ-oxo-bis[(p-methylphenoxo)(meso-tetraphenylporphyrinato)ruthenium(IV)] and ethoxo(meso-tetraphenylporphyrinato)(ethanol)ruthenium(III)-bisethanol. J Am Chem Soc 106(18):5151–63
Brown GM, Hopf FR, Ferguson JA, Meyer TJ, Whitten DG (1973) Metalloporphyrin redox chemistry. Effect of extraplanar ligands on the site of oxidation in ruthenium porphyrins. J Am Chem Soc 95(18):5939–42
Barley M, Becker JY, Domazetis G, Dolphin D, James BR (1981) Redox chemistry of ruthenium porphyrins: evidence for internal electron transfer and the characterization of [Ru(OEP+˙)] species. J Chem Soc Chem Comm 19:982–983. https://doi.org/10.1039/C39810000982
Connelly NG, Geiger WE (1996) Chemical redox agents for organometallic chemistry. Chem Rev 96(2):877–910. https://doi.org/10.1021/cr940053x
El-Attar MA, Xu N, Awasabisah D, Powell DR, Richter-Addo GB (2012) Cyclic voltammetric and fiber-optic infrared spectroelectrochemical studies of six-coordinate (por)Ru(NO)Cl compounds (por = porphyrinato dianion). Polyhedron 40(1):105–109. https://doi.org/10.1016/j.poly.2012.03.034
Acknowledgements
The authors thank the National Science Foundation (grant CHE-1726630) and the University of Oklahoma for funds to purchase the X-ray instrument and computers. The authors also thank the Fitchburg State University Special Projects Grant (grant T65-1230-T00-FCIN) and Fitchburg State University for funds for this project.
Author information
Authors and Affiliations
Contributions
D.A. performed experiments and wrote the main text of the manuscript. J.F.G. performed experiments and reviewed the manuscript. D.R.P. collected X-ray data, wrote the report and reviewed the manuscript. G.L. collected NMR data and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Awasabisah, D., Gangemi, J.F., Powell, D.R. et al. Preparation, characterization and electrochemical properties of ruthenium carbonyl octaethylporphyrins with axial quinoline and quinine ligands. Transit Met Chem 49, 75–86 (2024). https://doi.org/10.1007/s11243-023-00563-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-023-00563-6