Abstract
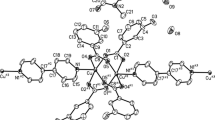
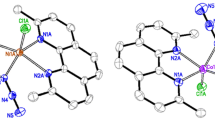
Dinuclear copper(II) complex [Cu2(L)2(μ2-1,1-N3)2(N3)2] (1) with double μ1,1-azido bridges and polynuclear nickel(II) complex [Ni(L)(μ2-1,1-N3)(μ2-1,3-N3)]n (2) with alternate double μ1,1-azido and μ1,3-azido bridges [L = 1-amino-2-(dimethylamino)ethane, N −3 = azide ion] have been synthesized and were characterized by physicochemical and spectroscopic methods. X-ray structural analysis revealed that each Cu(II) center of 1 adopts a distorted square-pyramidal geometry with a CuN5 chromophore ligated through two N atoms of L, two N atoms of double bridging (μ1,1-N3), and one N atom of terminal azide ion. On the other hand, each Ni(II) center around the asymmetric unit of 2 adopts a distorted octahedral geometry with a NiN6 chromophore ligated through two N atoms of L, two N atoms of double μ1,1-N3, and two N atoms of double μ1,3-N3 bridges. The adjacent nickel centers are connected to alternate double μ1,1-N3 and double μ1,3-N3 bridges, affording a one-dimensional (1D) polymeric chain structure. Temperature-dependent magnetic susceptibility measurements evidenced a dominant antiferromagnetic interaction between the metal centers of both complexes 1 and 2.









Similar content being viewed by others
References
Zoworotko MJ (2010) J Am Chem Soc 132:7821
Ricco R, Pfeiffer C, Sumida K, Sumby CJ, Falcaro P, Furukawa S, Champness NR, Doonan CJ (2016) Cryst Eng Comm 18:6532
Ozer RR, Hinestroza JP (2015) RSC Adv 5:15198
Mandal TN, Karmakar A, Sharma S, Ghosh SK (2018) Chem Rec 18(2):154
Wang ZN, Wang X, Wei SY, Wang JX, Bai FY, Xing YH, Sun LX (2015) New J Chem 39:4168
Mottillo C, Friscic T (2015) Chem Commun 51:8924
Chakrabarty R, Mukherjee PS, Stang PJ (2011) Chem Rev 111:6810
Neidig ML, Clark DL, Martin RL (2013) Coord Chem Rev 257:394
Reedijk J (2013) Chem Soc Rev 42:1776
Turnbull M M, Sugimoto T, Thompson L K (Eds) (1996) Molecular based magnetic materials: theory, techniques and applications n. 644 Washington: ACS symposium series ACS
Caneschi A, Gatteschi D, Pardi L, Sessoli R (1992) Clusters, chains and layered molecules: the chemist’s way to magnetic materials. In: Williams AF (ed) Perspectives in coordination chemistry. VCH, Weinheim
Long JR, Yang P (eds) (2003) Molecular cluster magnets in chemistry of nanostructured materials. World Scientific, Hong Kong
Escuer A, Aromí G (2006) Eur J Inorg Chem 23:4721–4736
Manson J L, Arif A M, Miller J S (1999) Chem Commun, 1479–1480
Zeng Y-F, Hu X, Liu F-C, Bu X-H (2009) Chem Soc Rev 38:469
Saha S, Mal D, Koner S, Bhattacharjee A, Gütlich P, Mondal S, Mukherjee M, Okamoto K-I (2004) Polyhedron 23:1811
Zbiri M, Saha S, Adhikary C, Chaudhuri S, Daul C, Koner S (2006) Inorg Chim Acta 359:1193
Adhikary C, Koner S (2010) Coord Chem Rev 254:2933
Lin J, Shen Z, Song Y, Xu H-J, Li Y-Z, You X-Z (2005) Inorg Chim Acta 358:1963
Youngme S, Chotkhun T, Leelasubcharoen S, Chaichit N, Pakawatchai C, van Albada GA, Reedijk J (2007) Polyhedron 26:725
Sun W-W, Qian X-B, Tian C-Y, Gao E-Q (2009) Inorg Chim Acta 362:2744
Li X-B, Ma Y, Zhang X-M, Zhang J-Y, Gao E-Q (2011) Eur J Inog Chem 2011:4738
Ray MS, Ghosh A, Bhattacharya R, Mukhopadhyay G, Drew MGB, Ribas J (2004) Dalton Trans, 252–259
Ruiz E, Cano J, Alvarez S, Alemany P (1998) J Am Chem Soc 120:11122
Sen S, Mitra S, Hughes DI, Rosair G, Desplanches C (2007) Polyhedron 26:1740
Boudreaux EA, Mulay LN (1976) Theory and applications of molecular paramagnetism. Wiley, New York
Bruker (2008) APEX2 (Version 2008.1–0), SAINT (Version 7.51A) and SADABS (Version 2007/4) Bruker AXS Inc. Madison, Wisconsin, USA.
Altomare A, Burla MC, Camalli M, Cascarano G, Giacovazzo C, Uagliardi A, Moliterni AGG, Polidori G, Spagna R (1999) J Appl Cryst 32:115
Sheldrick GM (2015) Acta Cryst C71:3
Nakamoto K (2009) Infrared and Raman spectra of inorganic and coordination compounds, part B, 6th edn. Wiley, New Jersey
Lever ABP (1984) Inorganic electronic spectroscopy, 2nd edn. Elsevier, New York
Reger DL, Debreczeni A, Smith M, Jezierska J, Ożarowski A (2012) Inorg Chem 51:1068
Chaudhuri P, Weyhermüler T, Bill E, Weighardt K (1996) Inorg Chim Acta 252:195
Addison AW, Rao TN, Reedjik J, van Rijn J, Verschoor CG (1984) J Chem Soc Dalton Trans, 1349–1356
Kahn O (1993) Molecular magnetism. VCH, New York
Acknowledgements
The work is financially supported by the Department of Science and Technology, Government of India, by grant SR/S1/IC-13/2010 to C.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were reported by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chowdhury, H., Rizzoli, C. & Adhikary, C. Azido derivatives of dinuclear copper(II) and 1D polynuclear nickel(II) complexes containing an unsymmetrical bidentate amine: synthesis, crystalline architecture, and magnetic behavior. Transit Met Chem 46, 139–147 (2021). https://doi.org/10.1007/s11243-020-00430-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-020-00430-8