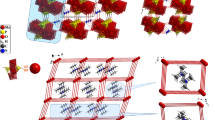
Abstract
The synthesis and characterization of fac-{tris[(E)-5-(hydroxymethyl)-2-methyl-4-((methylimino-κN)methyl)pyridin-3-olato-κO]amine}iron(III) 7.5-hydrate is described, together with its crystal structure at 95 K. The FeIII ion is six-coordinated in a trigonally distorted octahedral configuration with the hexadentate tripodal pyridoxal Schiff base ligand. A positional disorder of the pyridoxal hydroxymethyl group is identified, which partly undergoes a cyclization forming a five-membered ring with the imine moiety. The two configurations occur in the ratio 79.5:20.5. In the crystal, the hydrogen bonds and π–π stacking interactions expand the mononuclear units into 2D sheets which are further stabilized by a system of hydrogen bonds to the 3D supramolecular network. The magnetic susceptibility data and EPR measurements indicate that the octahedral Fe(III) centers exist as a mixture of low- and high-spin forms within the temperature range 2–300 K. The presence of a mixture of the majority high-spin and the minority low-spin components showing a spin transition is proposed. The effect of zero-field splitting on magnetic properties is discussed. EPR spectra at X-band display signals of a high-spin Fe(III) complex of the rhombohedral symmetry with the zero-field splitting parameters E/D ratio approximately 1/3, along with the signals of a low-spin Fe(III) complex. Temperature dependence of the EPR spectra revealed a bend temperature T ≈ 88 K attributed to spin transition of the minority Fe(III) centers. The thermal stability of the compound was investigated by (TG, DTG, DTA) techniques.
Graphical Abstract
New iron(III) complex with tripodal pyridoxal Schiff base ligand was prepared. Single-crystal XRD data revealed a positional disorder of a side hydroxymethyl group. The magnetic data were analysed using the spin Hamiltonian including the ZFS term. EPR measurements at X-band confirmed magnetic anisotropy of the complex. Thermal stability of the complex was investigated by (TG, DTG, DTA) techniques.













Similar content being viewed by others
References
Metzler DE, Snell EE (1952) Some transamination reactions involving Vitamin B 16 . J Am Chem Soc 74:979–983. https://doi.org/10.1021/ja01124a033
Christensen HN (1957) Metal chelates of pyridoxylidene amino acids. J Am Chem Soc 79:4073–4078
Heinert D, Martell AE (1963) Pyridoxine and pyridoxal analogs. VIII. Synthesis and infrared spectra of metal chelates. J Am Chem Soc 85:1334–1337
Henderson W, Koh L, Ranford J, Robinson W, Svensson J, Vittal J, Wang Y, Xu Y (1999) Clusters in bis-tridentate tethered domains of an iron chelating drug [dagger]. J Chem Soc Dalton Trans 19:3341–3343. https://doi.org/10.1039/A904728J
Pishchugin FV, Tuleberdiev IT (2010) Effect of structure of the amino acids and amines on the rate and mechanism of their condensations with pyridoxal. Russ J Gener Chem 80:1836–1840. https://doi.org/10.1134/s1070363210090203
Back DF, Manzoni de Oliveira G, Schulz Lang E, Vargas JP (2008) Assembly of new Schiff base ligands derived from vitamin B6 and stabilization through complexation of N, N′-bis-(pyridoxylideneimine)-o-phenylene: synthesis and X-ray structural features of pyridoxal/o-phenylenediamine adducts and of [UO2(H2pyr2phen)Cl]NO3. Polyhedron 27:2551–2556. https://doi.org/10.1016/j.poly.2008.05.006
Dutta Banik S, Chandra A (2014) A hybrid QM/MM simulation study of intramolecular proton transfer in the pyridoxal 5′-phosphate in the active site of transaminase: influence of active site interaction on proton transfer. J Phys Chem B 118:11077–11089. https://doi.org/10.1021/jp506196m
Casasnovas R, Frau J, Ortega-Castro J, Donoso J, Muñoz F (2013) C–H activation in pyridoxal-5′-phosphate and pyridoxamine-5′-phosphate Schiff bases: effect of metal chelation. A computational study. J Phys Chem B 117:2339–2347. https://doi.org/10.1021/jp311861p
Casas JS, Couce MD, Sordo J (2012) Coordination chemistry of vitamin B6 and derivatives: a structural overview. Coord Chem Rev 256:3036–3062. https://doi.org/10.1016/j.ccr.2012.07.001
Pandiarajan D, Ramesh R (2012) Suzuki–Miyaura cross-coupling reaction of aryl bromides catalyzed by palladium(II) pyridoxal hydrazone complexes. J Organomet Chem 708–709:18–24. https://doi.org/10.1016/j.jorganchem.2012.02.010
Manikandan R, Anitha P, Prakash G, Vijayan P, Viswanathamurthi P, Butcher RJ, Malecki JG (2015) Ruthenium(II) carbonyl complexes containing pyridoxal thiosemicarbazone and trans-bis(triphenylphosphine/arsine): synthesis, structure and their recyclable catalysis of nitriles to amides and synthesis of imidazolines. J Mol Catal A: Chem 398:312–324. https://doi.org/10.1016/j.molcata.2014.12.017
Miodrag GJ, Nebojša ZR, Branka BH, Mirjana ML, Miloš PS, Miloš BŽ (2014) Photoluminescence study of cobalt (III) and copper (II) complexes with the Schiff base of pyridoxal and aminoguanidine. Phys Scr 2014:14010
Rosu T, Pahontu E, Reka-Stefana M, Ilies D-C, Georgescu R, Shova S, Gulea A (2012) Synthesis, structural and spectral studies of Cu(II) and V(IV) complexes of a novel Schiff base derived from pyridoxal. Antimicrobial activity. Polyhedron 31:352–360. https://doi.org/10.1016/j.poly.2011.09.044
Iqbal MS, Khan AH, Loothar BA, Bukhari IH (2009) Effect of derivatization of sulfamethoxazole and trimethoprim with copper and zinc on their medicinal value. Med Chem Res 18:31–42. https://doi.org/10.1007/s00044-008-9104-5
Naskar S, Naskar S, Figgie HM, Sheldrick WS, Chattopadhyay SK (2010) Synthesis, crystal structures and spectroscopic properties of two Zn(II) Schiff’s base complexes of pyridoxal. Polyhedron 29:493–499. https://doi.org/10.1016/j.poly.2009.06.040
Baker E, Richardson D, Gross S, Ponka P (1992) Evaluation of the iron chelation potential of hydrazones of pyridoxal, salicylaldehyde and 2-hydroxy-1-naphthylaldehyde using the hepatocyte in culture. Hepatology 15:492–501. https://doi.org/10.1002/hep.1840150323
Gao J, Richardson DR (2001) The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents, IV: the mechanisms involved in inhibiting cell-cycle progression. Blood 98:842–850
Kalinowski DS, Sharpe PC, Bernhardt PV, Richardson DR (2007) Design, synthesis, and characterization of new iron chelators with anti-proliferative activity: structure − activity relationships of novel thiohydrazone analogues. J Med Chem 50:6212–6225. https://doi.org/10.1021/jm070839q
Yemeli Tido EW, Vertelman EJM, Meetsma A, van Koningsbruggen PJ (2007) Crystal structure and magnetic behaviour of a five-coordinate iron(III) complex of pyridoxal-4-methylthiosemicarbazone. Inorg Chim Acta 360:3896–3902. https://doi.org/10.1016/j.ica.2007.03.045
Yemeli Tido EW, Alberda van Ekenstein GOR, Meetsma A, van Koningsbruggen PJ (2008) Study of neutral Fe(III) complexes of pyridoxal-N-substituted thiosemicarbazone with desolvation-induced spin-state transformation above room temperature. Inorg Chem 47:143–153. https://doi.org/10.1021/ic701695b
Tido EWY, Faulmann C, Roswanda R, Meetsma A, van Koningsbruggen PJ (2010) Tuning of the charge in octahedral ferric complexes based on pyridoxal-N-substituted thiosemicarbazone ligands. Dalton Trans 39:1643–1651. https://doi.org/10.1039/B911114J
Said HM, Ortiz A, Ma TY (2003) A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: regulation by a PKA-mediated pathway. Am J Physiol Cell Physiol 285:C1219
Banerjee S, Dixit A, Shridharan RN, Karande AA, Chakravarty AR (2014) Endoplasmic reticulum targeted chemotherapeutics: the remarkable photo-cytotoxicity of an oxovanadium(iv) vitamin-B6 complex in visible light. Chem Commun 50:5590–5592. https://doi.org/10.1039/C4CC02093F
Sarkar T, Banerjee S, Hussain A (2015) Significant photocytotoxic effect of an iron(iii) complex of a Schiff base ligand derived from vitamin B6 and thiosemicarbazide in visible light. RSC Adv 5:29276–29284. https://doi.org/10.1039/C5RA04207K
Basu U, Pant I, Hussain A, Kondaiah P, Chakravarty AR (2015) Iron(III) complexes of a pyridoxal Schiff base for enhanced cellular uptake with selectivity and remarkable photocytotoxicity. Inorg Chem 54:3748–3758. https://doi.org/10.1021/ic5027625
Wani WA, Baig U, Shreaz S, Shiekh RA, Iqbal PF, Jameel E, Ahmad A, Mohd-Setapar SH, Mushtaque M, Ting Hun L (2016) Recent advances in iron complexes as potential anticancer agents. New J Chem 40:1063–1090. https://doi.org/10.1039/C5NJ01449B
Kuźnik N, Szafraniec-Gorol G, Oczek L, Grucela A, Jewuła P, Kuźnik A, Zassowski P, Domagala W (2014) A study on the synthesis and properties of substituted EHBG-Fe(III) complexes as potential MRI contrast agents. J Organomet Chem 769:100–105. https://doi.org/10.1016/j.jorganchem.2014.07.011
Mossin S, Tran BL, Adhikari D, Pink M, Heinemann FW, Sutter J, Szilagyi RK, Meyer K, Mindiola DJ (2012) A mononuclear Fe(III) single molecule magnet with a 3/2⟷5/2 spin crossover. J Am Chem Soc 134:13651–13661. https://doi.org/10.1021/ja302660k
Martell Arthur E (1968) Catalytic effects of metal chelate compounds. Pure Appl Chem 17:129
Mukherjee A, Dhar S, Nethaji M, Chakravarty AR (2005) Ternary iron(II) complex with an emissive imidazopyridine arm from Schiff base cyclizations and its oxidative DNA cleavage activity. Dalton Trans 2:349. https://doi.org/10.1039/b415864d
Kumar A, Kumar D (2008) Synthesis and antimicrobial activity of some metal complexes derived from 2-(27′-hydroxyphenyl)benzoxazole. Asian J Chem 20:1588
Lehnert N, Ho RYN, Que L, Solomon EI (2001) Electronic structure of high-spin iron(III) − alkylperoxo complexes and its relation to low-spin analogues: reaction coordinate of O − O bond homolysis. J Am Chem Soc 123:12802–12816. https://doi.org/10.1021/ja011450+
Hayami S, Matoba T, Nomiyama S, Kojima T, Osaki S, Maeda Y (1997) Structures and magnetic properties of some Fe(III) complexes with hexadentate ligands: in connection with spin-crossover behavior. Bull Chem Soc Jpn 70:3001–3009. https://doi.org/10.1246/bcsj.70.3001
Sharpe AG, Housecroft C (2012) Inorganic chemistry. Pearson Education, New York
Cotton FA, Herrero S, Jiménez-Aparicio R, Murillo CA, Urbanos FA, Villagrán D, Wang X (2007) How small variations in crystal interactions affect macroscopic properties. J Am Chem Soc 129:12666–12667. https://doi.org/10.1021/ja075808z
Jana S, Bhattacharyya A, Ghosh BN, Rissanen K, Herrero S, Jiménez-Aparicio R, Chattopadhyay S (2016) Synthesis, characterization and magnetic study of two new octahedral iron(III) complexes with pendant zwitterionic Schiff bases. Inorg Chim Acta 453:715–723. https://doi.org/10.1016/j.ica.2016.09.005
Phonsri W, Harding P, Liu L, Telfer SG, Murray KS, Moubaraki B, Ross TM, Jameson GNL, Harding DJ (2017) Solvent modified spin crossover in an iron(iii) complex: phase changes and an exceptionally wide hysteresis. Chem Sci 8:3949–3959. https://doi.org/10.1039/C6SC05317C
Boča R (2004) Zero-field splitting in metal complexes. Coord Chem Rev 248:757–815. https://doi.org/10.1016/j.ccr.2004.03.001
Boča R (2012) A handbook of magnetochemical formulae. Elsevier, Amsterdam
Atkins PW, De Paula J (2014) Atkins’ physical chemistry, 10th edn. Oxford University Press, Oxford
Pilbrow JR (1990) Transition ion electron paramagnetic resonance. Clarendon Press, Oxford. ISBN 0-19-855214-9
Stoll S, Schweiger A (2006) EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J Magn Reson 178:42–55. https://doi.org/10.1016/j.jmr.2005.08.013
Abragam A, Bleaney B (1970) Electron paramagnetic resonance of transition ions. Clarendon Press, Oxford
Fallon GD, McLachlan GA, Moubaraki B, Murray KS, O’Brien L, Spiccia L (1997) Mononuclear chromium(III), manganese(II) and iron(III) complexes of the pentadentate ligand 1,4-bis(2-pyridylmethyl)-1,4,7-triazacyclononane. J Chem Soc, Dalton Trans. https://doi.org/10.1039/A700756F
Gütlich P, Gaspar AB, Garcia Y (2013) Spin state switching in iron coordination compounds. Beilstein J Org Chem 9:342–391. https://doi.org/10.3762/bjoc.9.39
Nihei M, Shiga T, Maeda Y, Oshio H (2007) Spin crossover iron(III) complexes. Coord Chem Rev 251:2606–2621. https://doi.org/10.1016/j.ccr.2007.08.007
Burla MC, Camalli M, Carrozzini B, Cascarano GL, Giacovazzo C, Polidori G, Spagna R (2003) SIR2002: the program. J Appl Crystallogr 36:1103
Petříček V, Dušek M, Palatinus L (2014) Crystallographic computing system JANA2006: general features. Z Krist Cryst Mater 229:345
Brandenburg K, Putz H (1999) DIAMOND. Crystal Impact GbR, Bonn
Kuhs W (1992) Generalized atomic displacements in crystallographic structure analysis. Acta Crystallogr Sect A 48:80–98. https://doi.org/10.1107/S0108767391009510
Acknowledgements
This work was supported by the Ministry of Education of the Czech Republic, Grant No. 20/2018 for specific university research. The crystallographic part was supported by the Project 15-12653S of the Czech Science Foundation using instruments of the ASTRA laboratory established within the Operation program Prague Competitiveness—Project CZ.2.16/3.1.00/24510. M. Buryi and V. Laguta thank for the financial support from the Ministry of Education, Youth and Sports of Czech Republic under Projects LO1409 and CZ.02.1.01/0.0/0.0/16_013/0001406. The authors would like to thank Jiří Šturala for his valuable comments and suggestions to elucidate the mechanism of synthesis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Murašková, V., Dušek, M., Buryi, M. et al. Synthesis, characterization and X-ray crystal structure of an iron(III) complex of a tripodal pyridoxal Schiff base ligand: effects of positional disorder on its magnetic properties. Transit Met Chem 43, 605–619 (2018). https://doi.org/10.1007/s11243-018-0249-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0249-x