Abstract
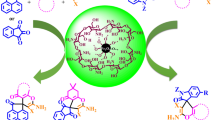
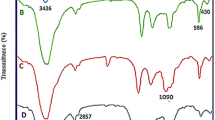
A magnetically recoverable biopolymer-based nanocatalyst was prepared through the covalent immobilization of a chitosan-bound 2-hydroxynaphthaldehyde Pd complex on the surface of superparamagnetic nanoparticles. The nanocatalyst was characterized by FTIR, X-ray powder diffraction and scanning electron microscopy, revealing an average particle size of 70 nm. The catalyst shows high thermostability by thermogravimetric analysis. Estimated Pd loading by inductively coupled plasma atomic emission analysis was found to be 0.348 mmol g−1. The nanocatalyst exhibits excellent catalytic performance in Suzuki couplings of various aryl halides with phenylboronic acid, and Heck reactions of iodo- and bromoarenes with butylacrylate. The catalyst can be easily separated from the reaction mixture with an external magnet and reused consecutively four times without significant loss in activity.









Similar content being viewed by others
References
Han FS (2013) Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: a remarkable advance from palladium to nickel catalysts. Chem Soc Rev 42:5270–5298
Polshettiwar V, Decottignies A, Len C, Fihri A (2010) Suzuki–Miyaura cross-coupling reactions in aqueous media: green and sustainable syntheses of biaryls. Chemsuschem 3:502–522
Alonso F, Beletskaya IP, Yus M (2008) Non-conventional methodologies for transition-metal catalysed carbon–carbon coupling: a critical overview. Part 2: the Suzuki reaction. Tetrahedron 64:3047–3101
Martin R, Buchwald SL (2008) Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc Chem Res 41:1461–1473
Mondal J, Modak A, Bhaumik A (2011) One-pot efficient Heck coupling in water catalyzed by palladium nanoparticles tethered into mesoporous organic polymer. J Mol Catal A Chem 350:40–48
Trzeciak AM, Ziołkowski JJ (2007) Monomolecular, nanosized and heterogenized palladium catalysts for the Heck reaction. Coord Chem Rev 251:1281–1293
Kalbasi RJ, Mosaddegh N, Abbaspourrad A (2012) Palladium nanoparticles supported on a poly(N-vinyl-2-pyrrolidone)-modified mesoporous carbon nanocage as a novel heterogeneous catalyst for the Heck reaction in water. Tetrahedron Lett 53:3763–3766
Polshettiwar V, Molnar A (2007) Silica-supported Pd catalysts for Heck coupling reactions. Tetrahedron 63:6949–6976
Phan NTS, Van Der Sluys M, Jones CW (2006) On the nature of the active species in palladium catalyzed Mizoroki–Heck and Suzuki–Miyaura couplings–homogeneous or heterogeneous catalysis, a critical review. Adv Synth Catal 348:609–679
Cho JH, Shaughnessy KH (2011) Aqueous-phase Heck coupling of 5-iodouridine and alkenes under phosphine-free conditions. Synlett. https://doi.org/10.1055/s-0031-1289886
Polshettiwar V, Varma RS (2009) Nanoparticle-supported and magnetically recoverable palladium (Pd) catalyst: a selective and sustainable oxidation protocol with high turnover number. Org Biomol Chem 7:37–40
Safari J, Javadian L (2014) Chitosan decorated Fe3O4 nanoparticles as a magnetic catalyst in the synthesis of phenytoin derivatives. RSC Adv 4:48973–48979
Cho SD, Kim HK, Yim HS, Kim MR, Lee JK, Kim JJ, Yoon YJ (2007) Suzuki–Miyaura coupling reaction of aryl chlorides using di(2,6 dimethylmorpholino)phenylphosphine as ligand. Tetrahedron 63:1345–1352
Alacid E, Nájera C (2008) First cross-coupling reaction of potassium aryltrifluoroborates with organic chlorides in aqueous media catalyzed by an oxime-derived palladacycle. Org Lett 10:5011–5014
Masllorens J, Gonzalez I, Roglans A (2007) Recoverable homogeneous palladium(0) catalyst for cross-coupling reactions of arenediazonium salts with potassium organotrifluoroborates: detection of catalytic intermediates by electrospray ionization mass spectrometry. Eur J Org Chem 1:158–166
Stevens PD, Fan J, Gardimalla HMR, Yen M, Gao Y (2005) Superparamagnetic nanoparticle-supported catalysis of Suzuki cross-coupling reactions. Org Lett 7:2085–2088
Lü B, Fu C, Ma S (2010) Application of a readily available and air stable monophosphine HBF4 salt for the Suzuki coupling reaction of aryl or 1-alkenyl chlorides. Tetrahedron Lett 51:1284–1286
Emmanuvel L, Sudalai A (2007) Phosphine ligand and base-free, Pd- catalyzed oxidative cross-coupling reaction of arylboronic acids with arylmercuric acetates. Arkivoc 14:126–133
Ma J, Cui X, Zhang B, Song M, Wu Y (2007) Ferrocenylimidazoline palladacycles: efficient phosphine-free catalysts for Suzuki-Miyaura cross-coupling reaction. Tetrahedron 63:5529–5538
Samarasimhars Eddy M, Prabhu G, Vishwanatha TM, Sureshbabu VV (2013) PVC-supported palladium nanoparticles: an efficient catalyst for Suzuki cross-coupling reactions at room temperature. Synthesis 45:1201–1206
Ramesh Kumar NSC, Victor Paul Raj I, Sudalai A (2007) Sulfonamide- and hydrazine-based palladium catalysts: stable and efficient catalysts for C–C coupling reactions in aqueous medium. J Mol Catal A Chem 269:218–244
Peng YY, Liu J, Lei X, Yin Z (2010) Room-temperature highly efficient Suzuki–Miyaura reactions in water in the presence of Stilbazo. Green Chem 12:1072–1075
Nandurkar NS, Bhanage BM (2008) Palladium bis(2,2,6,6-tetramethyl-3,5-heptanedionate) catalyzed Suzuki, Heck, Sonogashira, and cyanation reactions. Tetrahedron 64:3655–3660
Baia L, Wanga JX (2007) Reusable, polymer-supported, palladium-catalyzed, atom- efficient coupling reaction of aryl halides with sodium tetraphenylborate in water by focused microwave irradiation. Adv Synth Catal 350:315–320
Xu Q, Duan WL, Lei ZY, Zhu ZB, Shi M (2005) A novel cis-chelated Pd(II)–NHC complex for catalyzing Suzuki and Heck-type cross-coupling reactions. Tetrahedron 61:11225–11229
Aksın Ö, Türkmen H, Artok L, Ҫetinkaya B, Ni Ch, Büyükgüngör O, Özkal E (2006) Compromise between conjugation length and charge-transfer in nonlinear optical η5-monocyclopentadienyliron(II) complexes with substituted oligo-thiophene nitrile ligands: synthesis, electrochemical studies and first hyperpolarizabilities. J Organomet Chem 691:3027–3041
Mino T, Shibuya M, Suzuki S, Hirai K, Sakamoto M, Fujita T (2012) Palladium-catalyzed Mizoroki–Heck type reaction with aryl trialkoxysilanes using hydrazone ligands. Tetrahedron 68:429–432
Amali AJ, Rana RK (2009) Stabilisation of Pd(0) on surface functionalised Fe3O4 nanoparticles: magnetically recoverable and stable recyclable catalyst for hydrogenation and Suzuki–Miyaura reactions. Green Chem 11:1781–1786
Dhara K, Sarkar K, Srimani D, Saha SK, Chattopadhyaye P, Bhaumik A (2010) A new functionalized mesoporous matrix supported Pd(II)-Schiff base complex: an efficient catalyst for the Suzuki–Miyaura coupling reaction. Dalton Trans 39:6395–6402
Ma H, Cao W, Bao Zh, Lei Z (2012) Biopolymer–metal complex wool–Pd as a highly active catalyst for Suzuki reaction in water. Catal Sci Technol 2:2291–2296
Jin X, Zhang K, Sun J, Wang J, Dong Z, Li R (2012) Magnetite nanoparticles immobilized Salen Pd (II) as a green catalyst for Suzuki reaction. Catal Commun 26:199–203
Liu H, Wang P, Yang H, Niu J, Ma J (2015) Palladium supported on hollow magnetic mesoporous spheres: a recoverable catalyst for hydrogenation and Suzuki reaction. New J Chem 39:4343–4350
Nasrollahzadeh M, Sajadi SM, Maham M (2015) Green synthesis of palladium nanoparticles using Hippophaerhamnoides Linn leaf extract and their catalytic activity for the Suzuki–Miyaura coupling in water. J Mol Catal A Chem 396:297–303
Yuan D, Chen L, Yuan L, Liao S, Yang M, Zhang Q (2016) Superparamagnetic polymer composite microspheres supported Schiff base palladium complex: an efficient and reusable catalyst for the Suzuki coupling reactions. Chem Eng J 287:241–251
Wang P, Liu H, Liu M, Li R, Ma J (2014) Immobilized Pd complexes over HMMS as catalysts for Heck cross-coupling and selective hydrogenation reactions. New J Chem 38:1138–1143
Shang N, Gao S, Zhou X, Feng C, Wang Z, Wang C (2014) Palladium nanoparticles encapsulated inside the pores of a metal–organic framework as a highly active catalyst for carbon–carbon cross-coupling. RSC Adv 4:54487–54493
Søbjerg LS, Gauthier D, Lindhardt AT, Bunge M, Finster K, Meyer RL, Skrydstrup T (2009) Bio-supported palladium nanoparticles as a catalyst for Suzuki–Miyaura and Mizoroki–Heck reactions. Green Chem 11:2041–2046
Sarkar SM, Rahman ML, Yusoff MM (2015) Heck, Suzuki and Sonogashira cross-coupling reactions using ppm level of SBA-16 supported Pd-complex. New J Chem 39:3564–3570
Liu G, Hou M, Song J, Jiang T, Fan H, Zhang Z, Han B (2010) Immobilization of Pd nanoparticles with functional ionic liquid grafted onto cross-linked polymer for solvent-free Heck reaction. Green Chem 12:65–69
Tamami B, Farjadian F, Ghasemi S, Allahyari H (2013) Synthesis and applications of polymeric N-heterocyclic carbene palladium complex-grafted silica as a novel recyclable nano-catalyst for Heck and Sonogashira coupling reactions. New J Chem 37:2011–2018
Cao M, Wei Y, Gao S, Cao R (2012) Synthesis of palladium nanocatalysts with cucurbit[n]uril as both a protecting agent and a support for Suzuki and Heck reactions. Catal Sci Technol 2:156–163
Acknowledgements
The authors appreciatively acknowledge the funding support received from the Ilam University, Ilam, Iran, on this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fakhri, A., Naghipour, A. Organometallic polymer-functionalized Fe3O4 nanoparticles as a highly efficient and eco-friendly nanocatalyst for C–C bond formation. Transit Met Chem 43, 463–472 (2018). https://doi.org/10.1007/s11243-018-0233-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0233-5