Abstract
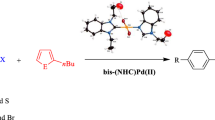
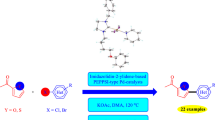
Palladium-catalyzed direct arylation of heteroaromatics has become a popular method for producing carbon–carbon bonds via C–H bond activation. A wide diversity of heteroaromatics such as furan, thiophenes and thiazoles can be used for this reaction. This paper reports the synthesis of N-propylphthalimide-substituted bis-(NHC)PdX2 complexes (NHC = N-heterocyclic carbene), and their catalytic activity in direct arylation reactions. The complexes have been prepared from Ag(I)NHC precursors by transmetallation and characterized by spectroscopy and elemental analysis. The bis-(NHC)PdX2 complexes show excellent activity as catalysts in the direct arylation reactions of 2-n-butylfuran, 2-n-butylthiophene and 2-isopropylthiazole.




Similar content being viewed by others
References
Kakiuchi F, Murai S (2002) Acc Chem Res 35:826
Miura M, Nomura M (2002) Top Curr Chem 219:211
Kakiuchi F, Chatani N (2003) Adv Synth Catal 345:1077
Alberico D, Scott ME, Lautens M (2007) Chem Rev 107:174
Sun L, Li H, Yu D-G, Yu M, Zhou X, Lu X-Y, Huang K, Zheng S-F, Li B-J, Shi Z-J (2010) Nat Chem 2:1044
Liu W, Cao H, Lei A (2010) Angew Chem Int Ed 49:2004
Stanforth SP (1998) Tetrahedron 54:263
Hassan M, Se’vignon M, Gozzi C, Shulz E, Lemaire M (2002) Chem Rev 102:1359
Miura M, Nomura M (2002) Top Curr Chem 219:211
Kakiuchi F, Chatani N (2003) Adv Synth Catal 345:1077
Alberico D, Scott ME, Lautens M (2007) Chem Rev 107:174
Yokooji A, Satoh T, Miura M, Nomura M (2004) Tetrahedron 60:6757
Campeau L-C, Rousseaux S, Fagnou K (2005) J Am Chem Soc 127:18020
Do H-Q, Khan RMK, Daugulis O (2008) J Am Chem Soc 130:15185
Liégault B, Lapointe D, Caron L, Vlassova A, Fagnou KJ (2009) Org Chem 74:1826
Ueda K, Yanagisawa S, Yamaguchi J, Itami K (2010) Angew Chem Int Ed 49:8946
Tamba S, Fuji R, Mori A, Hara K, Koumura N (2011) Chem Lett 40:922
Fu HY, Chen L, Doucet H (2012) J Org Chem 77:4473
Reddy VP, Qiu R, Iwasaki T, Kambe N (2013) Org Lett 15:1290
Bheeter CB, Chen L, Soulé J-F, Doucet H (2016) Catal Sci Technol 6:2005
Yuan K, Doucet H (2013) Chem Cat Chem 5:3495
Tsurugi H, Yamamoto K, Nagae H, Kaneko H, Mashima K (2014) Dalton Trans 43:2331
Khake SM, Jagtap RA, Dangat YB, Gonnade RG, Vanka K, Punji B (2016) Organometallics 35:875
Abdellaoui F, Youssef C, Ammar HB, Roisnel T, Soulé J-F, Doucet H (2016) ACS Catal 6:4248
Akkoç S, Gök Y (2015) Inorg Chim Acta 429:34
Yiğit M, Yiğit B, Gök Y (2016) Inorg Chim Acta 453:23
Lin N-C, Syu HJH, Naziruddin AR, Liu F-C, Lin IJB (2017) RSC Adv 7:11652
Herrmann WA (2002) Angew Chem Int Ed 41:1290
Peris E, Crabtree RH (2004) Coord Chem Rev 248:2239
Burling S, Whittlesey MK, Williams JMJ (2005) Adv Synth Catal 347:591
Zhang CM, Trudell ML (2000) Tetrahedron Lett 41:595
Akkoç S, Gök Y (2014) Appl Organometal Chem 28:854
Sarı Y, Aktaş A, Barut Celepci D, Gök Y, Aygün M (2017) Catal Lett 147:2340
Gök Y, Akkoç S, Erdoğan H, Albayrak S (2016) J Enzym Inhib Med Chem 31:1322
Yiğit M, Yiğit B, Gök Y (2016) Inorg Chim Acta 453:23
Acknowledgements
This work was financially supported by İnönü University Research Fund (IUBAP 2012/02) and the authors acknowledge İnönü University Scientific and Technology Center for the elemental analyses of the compounds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erdoğan, H., Aktaş, A., Gök, Y. et al. N-Propylphthalimide-substituted bis-(NHC)PdX2 complexes: synthesis, characterization and catalytic activity in direct arylation reactions. Transit Met Chem 43, 31–37 (2018). https://doi.org/10.1007/s11243-017-0190-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-017-0190-4