Summary
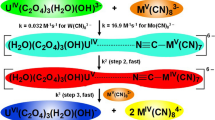
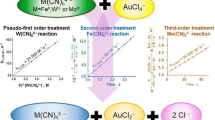
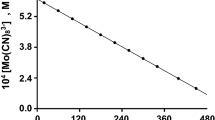
The kinetics of oxidation of [Mo(CN)8]4− by IO −4 in aqueous acid is described by the equation: d[{Mo(CN)8}3−]/ dt=2k3[{Mo(CN)8}4−][IO −4 ][H+]. Unlike IO −4 oxidations of [Fe(CN)6]4− and [W(CN)8]4−, no [H+] independent term exists in the [Mo(CN)8]4− reaction, which indicates that, in neutral and alkaline solutions, oxidation of [Mo(CN)8]4− is thermodynamically unfavourable. An inner-sphere mechanism, consistent with the rate law, is proposed. This conclusion is based, in the absence of direct evidence, on the observed behaviour of IO −4 as an inner-sphere oxidant.
Similar content being viewed by others
References
Y. Sulfab and A. I. Abu-Shadi,Inorg. Chim. Acta, 21, 115 (1977).
A. Y. Kasim and Y. Sulfab,Inorg. Chim. Acta, 24, 247 (1977.
F. R. El-Eziri and Y. Sulfab,Inorg. Chim. Acta, 25, 15 (1977).
A. A. Abd El-Khalek and Y. Sulfab,J. Inorg. Nucl. Chem., in press (1981).
Y. Sulfab,J. Inorg. Nucl. Chem., 38, 2270 (1976).
A. Y. Kassim and Y. Sulfab,Inorg. Chem., 20, 506 (1981).
P. Guardado, A. Maestra and M. Baton,J. Inorg. Nucl. Chem., 43, 1392 (1981).
I. Pechet and Z. Luz,J. Am. Chem. Soc., 87, 4068 (1965).
A. Indelli, F. Ferranti and F. Secco,J. Phys. Chem., 70, 631 (1966).
G. J. Buist, in C. H. Bamford and C. F. H. Tripper, (Eds.),Comprehensive Chemical Kinetics, Elsevier,Vol. 6, pp. 435.
J. van der Poel and H. M. Neumann,Inorg. Synth., 1, 53 (1968).
M. H. Ford-Smith and J. H. Rawsthorne,J. Chem. Soc. A, 160 (1969).
F. S. H. Head and H. A. Standing,J. Chem. Soc., 1457 (1952).
R. P. Mitra, B. K. Sharma and H. Mohan,Aust. J. Chem., 25, 499 (1972).
H. A. Mottola and H. Freiser,Anal. Chem., 39, 1294 (1967).
A. Y. Kasim and Y. Sulfab,Inorg. Chim. Acta, 22, 169 (1977).
W. Latimer,Oxidation Potentials, Prentice-Hall, Englewood Cliffs, N. J., 2nd Edit. 1952.
R. J. Campion, N. Purdie and N. Sutin,Inorg. Chem., 3, 1091 (1964).
S. H. Laurie, J. M. Williams and C. J. Nyman,J. Phys. Chem., 68, 1311 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hussein, M.A., Sulfab, Y. Kinetics of the oxidation of octacyanomolybdate(IV) by periodate in aqueous acid. Transition Met Chem 7, 181–183 (1982). https://doi.org/10.1007/BF01035838
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01035838