Abstract
The date palm, or Phoenix dactylifera L., is one of the world’s oldest fruit trees. The entire date palm tree is used to produce a variety of goods, including food, clothing, fibre, and shelter. Tissue culture is one of the most recent methods used to multiply date palms and produce disease-free offspring. Plant tissue culture technology has advantages over conventional methods of propagation for the quick and extensive multiplication of significant plants in vitro, regardless of the season, and disease-free. This is because it preserves space and time. For this study, three different cultivars were used (Barhy, Sakkoti, and Shamia). For the three cultivars, ¾ MS medium supplemented with 1 mg/L indole butyric acid IBA and 0.25 mg/l activated charcoal (AC) provided the optimal in vitro culture conditions. Plant gene function research and cultivar development are now both possible thanks to the advancement of plant transformation technologies. Date palm plants were given the AT1G12660 “Thio-60” gene to make them resistant to fungus infection. Utilizing chitosan nanoparticles for genetic transformation, the gene was introduced into three cultivars of dates (Barhy, Sakkoti, and Shamia). Run a conventional PCR to verify genetic fusion into all three cultivars. The fungal infection with Fusarium oxysporum was used to determine the resistance of the transgenic cultivar lines after it was established that the thionin gene had been transferred into transgenic date palm cultivars. Date crop transgenic lines showed strong resistance and a decline in the percentage of fungal infection-induced inhibition.
Key message
Phoenix dactylifera in vitro culture was optimized on MS medium supplemented with phytohormones. Chitosan nanoparticle was applied for thionin genetic transformation into different cultivars to be resistant to Fusarium infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The monocotyledon Phoenix dactylifera L., which has a long lifespan and is a member of the Arecaceae family, is grown for its delectable fruit and for other reasons. Due to its long history of fruit production, its precise origin is unknown, but it most likely started in a North African desert oasis or even Southwest Asia. Some claim that it was first developed in Bahrain or Saudi Arabia, while others assert that Babylon, Iraq, was where it was first created. It was being produced as early as 6,000 BC in ancient Egypt and Mesopotamia, and it is also thought to have originated in the Arabian Gulf. Eastern Arabia had farming going on by 4,000 BC, according to archaeological discoveries. Later, dates were disseminated by Arabs throughout northern Africa and into Spain, while dates were first brought to California by Spaniards in 1765 near Mission San Ignacio (Abdelmonem and Rasmy 2007).
The date palm is a dioecious plant species with a large genetic diversity. Size, form, colour, and flavour of the fruit on the majority of female date palm cultivars may be used to identify it. Date palm genotype identification is a complex empirical activity based on physical traits (Al-Khalifah et al. 2012). Fruit and palm oil, both of which are widely used in the food industry, are both made by date palms (Phoenix dactylifera L.). It can be difficult to repopulate obstinate date palm genotypes using somatic embryogenesis or organogenesis in tissue culture techniques. However, micropropagation provides a way to produce an adequate number of superior, disease-free, and true-to-type plants to satisfy local and international markets (Khokhar and Teixeira da Silva 2017).
Plant tissue culture incorporates techniques and procedures used in a variety of botanical research fields and has a number of useful objectives (George et al. 2008). Palms are a very underutilised plant category when it comes to the use of biotechnology and genetic engineering for their improvement. Applications of modern biotechnologies to plant molecular biology and plant tissue culture can immediately help (Pourhosseini et al. 2013). The greatest method for choosing and propagating new, improved cultivars is tissue culture, which is becoming more and more important as the date palm genome is uncovered through study. The preservation of date palm germplasm has a significant role to play for tissue culture as well. Through in vitro cryopreservation, tissue culture material may be kept for a long time (Johnson 2011).
Chitosan nanoparticles can alter the structural makeup of proteins associated with tight junctions and increase transmucosal permeability, both of which promote the transport of the nanoparticles along their paracellular pathway (Peppas and Huang 2004). More recently, chitosan nanoparticle-based methods for transforming and introducing foreign genes into different tissues have been tested. Chitosan was identified as a cationic alkaline polysaccharide that occurs naturally, a stable cationic polymer, and a non-viral vector (Li et al. 2015; Tawfik and Ahmed 2022).
In varying degrees, illnesses and pests pose a persistent threat to date fruit production and, in some cases, the life of the trees themselves, depending on the region. Two diseases that harm date palms are particularly prevalent nowadays among their many ailments. The lethal vascular wilt known as Bayoud disease is brought on by a fungus that lives in the soil (Fusarium oxysporum) Ref. Late in the nineteenth century, this illness initially occurred in Morocco, where it has since spread significantly throughout the nation and into neighboring Algeria. It infects the highly valued Moroccan Medjool cultivar, and it spreads mostly through the movement of offshoots. The demise of the palm occurs many months after the first signs of leaf withering. The sole known biological or chemical control is cultivating disease-resistant cultivars, which appears to be the only current answer (Zaid and Arias-Jimenez 2002).
The main aims for this work were, first, to optimize the culture conditions of Dates cultivars which is a big problem to obtain in vitro; whereas the second, was to produce antifungal transgenic Dates’ cultivars to face the problem of the in vitro culture contamination. By producing thionin proteins from thio-60 gene, we have date palm plants resistance to fungi. Using chitosan nanoparticles, the thionin gene was introduced into the tissues of the date palm and the transformation was confirmed into the transgenic date palm cultivars. The antimicrobial activity of transgenic lines affirmed the resistance of transgenic plants against Fusarium oxysporum.
Materials and methods
Plant materials and location
The research work was conducted at Labs of Ain Shams Agricultural Center for Genetic Engineering and Biotechnology (ACGEB) and “Vitro Plant Labs” (Plant Tissue Culture Specialists), Egypt during the period between 2017 and 2022. All the chemicals and reagents utilized were of analytical or molecular grade.
Date palm cultivars: Three date palm cultivars (Phoenix dactylifera) (Barhy, Sakkoti, and Shamia) were brought from The Central Laboratory for Research and Development of Date Palms; Agricultural Research Centre, Giza, Egypt.
Methods
In vitro culture conditions and media
A laminar airflow-hood (Streamline®, made in Singapore) was used in all procedures of tissue culturing of date palm cultivars. The hood was fully equipped with a single U.V. lamp (15 W). The transfer area was also fitted with an air condition unit (Sharp®, 1.5 horse). A growth chamber was used as a growing area (5.20 × 4.80 m in length and width, respectively) containing 25 stands each of them has four shelves with two lamps (15 W fluorescent cool light) fitted 30 cm above the shelf. To control the temperature, two air condition units (Carrier®, 3.5 horse) were fitted in the growth chamber. Cultures of all experiments were incubated at 28 ± 2 °C and exposed to 2000–2500 lx for 16 h light using fluorescent lamps (2 lamps per shelf) alternated with 8 h of darkness. Each treatment comprised of three replicates with three explants per jar (50 ml) for each replicate. The initial culture medium used for the in vitro culture was the Murashige and Skoog basal nutrition medium (MS) (Murashige and Skoog 1962). The medium was supplemented with agar (6 g/l) to solidify the medium. The medium was adjusted to 5.7 pH and autoclaved for 20 min at 121 °C and 1.2 Kg/cm2 before being used.
Establishment of in vitro culture conditions
Explants (8 months, inform of cluster) were collected from 3 date palm’s cultivars (Phoenix dactylifera L.; Barhy, Sakkoti, and Shamia). Then, the collected explants were defoliated and cleaned with liquid soap and rinsed with a continuous flow of tap water for an hour. Explants were then moved to the laminar-flow cabinet under aseptic conditions. Sterilized explants were excised to 1-1.5 cm, each contain single lateral bud, cultured on different media strength to select the best concentration of medium solutes. Then the new generated plantlets were subjected to be cultured in MS medium at ¾ strength (3.3 g/l) as the best concentration.
Multiplication stage experiment
Defoliated micro shoots (2 cm) resulting from the establishment stage was divided into parts containing forming auxiliary buds or organogenesis and was taken for the multiplication experiment. They were cultured singly in the multiplication medium (¾ MS) which was supplemented with cytokinins using 6-Benzylaminopurine (BAP) at 1 mg/l and both supplemented with Kinetin (Kin) at 0.2 mg/l and activated charcoal was supplied 0.2 g/l.
Rooting stage experiment
Micro shoots (8 cm) obtained from the multiplication stage were transformed to a rooting medium (¾ MS) that was supplemented with IBA at a ratio of 0 to 0.5, 1and 1.5 mg/l. In addition, activated charcoal was supplied at 0.00, 0.25, 0.50 and 0.75 g/l as well as ¾ MS free of growth hormones (zero-level control). Re-culture was carried out every five weeks interval for three times.
Thionin gene manipulation
A prepared recombinant plasmid (pMiniT) containing the thio-60 gene was supplied from Faculty of Science – Helwan University. The plasmid was conserved in DH5-α bacterial glycerol stock. A bacterial sub-culture was performed for amplification of the recombinant plasmid on LB broth media supplemented with ampicillin antibiotic (100 µg/ml). A plasmid mini-prep protocol was applied to isolate the modified plasmid using alkaline lysis method according to the protocol of Maniatis et al. (1982).
Chitosan nanoparticle preparation and transformation
In this study, chitosan nanoparticles were utilized for transformation of thionin gene (Thio-60) into 3 date’s cultivars plants (Barhy, Sakkoti, and Shamia). Mansoori et al. (2006) characterized the CS/pDNA production procedure as follows: CSNPs were dissolved in 25 mM acetic acid, which was subsequently adjusted to pH 5.5 at a final concentration of 0.08%. The CS and recombinant plasmid were first incubated for 15 min in a water bath at 55oC. The CS/pDNA complexes were then formed in an equal volume, followed by 1 min of intensive swirling on a vortex mixer.
Abdel-Razik et al. (2017) and Hussien (2020) established a technique for chitosan nanoparticle genetic transformation into plant tissue: plantlets of dates cultivars (8–23 cm tall) were injected at the plumule area with a syringe carrying a CS/pDNA complex. To regenerate plants, the explants (injected seedlings) were then moved to MS medium supplemented with (2 mg/l BAP and 1 mg/l kin) hormones and 100 g/lampicillin. This process was place over the course of a 4-week period at 25 °C.
Molecular characterization of putative transgenic dates lines
Using the Cetyl-Trimethyl Ammonium Bromide (CTAB) technique, total genomic DNAs were isolated from the leaves of putative transformed and untransformed date palm plants (Rogers and Bendich 1985). Using whole genomic DNA as templates, DNA fragments from using a set of primers, the Thio-60 transgenes was amplified by PCR composed of 35 cycles with 60oC of annealing temperature.
Morphological parameters
Different six morphological parameters were measured in both putative transgenic and non-transgenic lines derived from the three palms cultivar. These parameters were estimated to measure the effect of transformation on the morphological behavior of these plants. These parameters include fresh weight (g), shoot length (cm), leaf number, root length (cm), root number and rooting percentage (%). The rooting percentage is an indication for the number of individuals producing roots comparing to the total number of individuals in all jars.
Pathogenicity test
This bioassay was applied to test fungal resistance of putative transgenic date palm lines expressing thionin against the phytopathogenic fungi (Fusarium oxysporum). Spore suspension was prepared by immersing fungal discs in 5 ml of sterile distilled water to release the spores. The spores were collected with a sterile Pasteur pipette, and their concentration was adjusted to 2 × 105 spores/ml using sterile water. This assay was applied to detached leaves of transgenic and control plants of palms cultivars. Detached leaves from mature putative transgenic and non-transgenic date palm plants, grown in vitro for 4–5 weeks, were placed in a Petri dish with wet filter paper, wounded in the middle on both sides of the midrib, and inoculated with the spore suspension (20 µl each). After inoculation, this incubated at room temperature under 16 h. light/8 hrs. dark conditions and high humidity for a week. Pictures were taken 5 days after inoculation (Khan et al. 2008).
Statistical analysis
All morphological parameters were set up using three replications of a fully randomized complete block design. All the parameters were measured in ten replicates after the third sub-culture. The acquired data were put through a test of analysis of variance in SPSS 21 (Shuaib et al. 2007).
Results
The in vitro culture of different three cultivars of palms (Barhy, Sakkoti, and Shamia) was optimized. The thionin genes (Thio-60), it is belonged to PR-13 family (defense protein) which isolated from Arabidopsis thaliana. It was transferred into plants of the three date palm cultivars (Phoenix dactylifera L.). Chitosan nanoparticle was used in this study as a genetic transformation method. Successful transformation into date palm cultivars was detected by successful amplification of inserts using PCR reaction. Besides, spore suspension of Fusarium oxysporum was applied to the detached leaves of in vitro culture of both putative transgenic and non-transgenic lines.
Plant materials and growth conditions
Shoots of three date palm cultivars (Barhy, Sakkoti, and Shamia) were regenerated on MS media with different media strength (solutes concentration) to select the best concentration. The concentration with the highest parameters was ¾ (3.3 g/l) (Table 1). The regeneration of these plants continued for three years to transforming the thionin gene carried out. Figure (1) illustrated the shapes of regenerated shoots of the three date palm cultivars. The optimization of the in vitro culture conditions was illustrated in Tables (2, 3, 4) for Barhy, Sakkoti and Shamia cultivars, respectively. The results showed that the best treatment to obtain the highest culture conditions was ¾ MS supplemented with 1 mg/l IBA and 0.25 mg/l activated charcoal. For all cultivars, the shoot number is fixed to one branch, and the rooting percentage is fixed to 100% except for the control, no rooting observed.
The measured six morphological parameters of both putative transgenic and non-transgenic dates cultivars lines were analyzed and recorded in Table (5). It was noticed that transgenic lines were higher in the measured parameters especially number of roots which is highly stimulated in putative transgenic lines.
Gene analysis
The transformation and presence of thio-60 gene in genetic materials of putative transgenic date palm lines was detected in the plant of the 3 date’s cultivars using conventional PCR technique. Transformation succussed in the three dates cultivars’ lines (Barhy, Sakkoti, and Shamia). The PCR for non-transgenic lines (control) confirmed the absence of thio-60 gene (Fig. 2).
Agarose gel electrophoresis for detection of thio-60 gene in the putative transgenic cultivars, lines of dates. M: 1 kb ladder; C: control gene from Arabidopsis thaliana; (1) transgenic Barhy, (2) transgenic Sakkoti, (3) transgenic Shamia, (4) non-transgenic Barhy, (5) non-transgenic Sakkoti, (6) non-transgenic Shamia
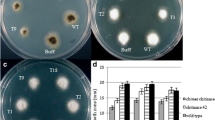
Pathogenicity test
It is qualitative and quantitative descriptions for the symptoms resulted from spore suspension of Fusarium oxysporum on date’s detached leaf to assess the resistance of dates’ cultivars of putative transgenic plants in compared to non-transgenic plants. The obtained results are summarized in histogram in Fig. (3).
Discussion
The date palm, Phoenix dactylifera L., is an important part of the reclamation effort and is crucial to Egyptian agriculture. One of the most modern techniques used for date palm (Phoenix dactylifera L.) multiplication and manufacture of disease-free offshoots is tissue culture, cell suspension culture, and artificial seeds. Additionally, it allows for the multiplication of date palms, which was previously impossible. At first, date palm is usually reproduced through a small number of offshoots that are developed in a palm tree. The success of date palm branch establishment varies among date palm varieties. Most current transformation procedures call for a tissue culture step to finally restore plants. In fact, many plant transformation systems are supported by the totipotency of plant cells. Somatic embryogenesis and organogenesis are the two processes used to regenerate plants from cell culture. Increased transformation efficiency is the consequence of improvements in transformation technologies and advancements in tissue culture. Several advantages of date palm micropropagation using tissue culture methods include: (1) large-scale commercial cultivar multiplication; (2) elite and carefully chosen cultivar propagation with desirable traits (such as resistance to Bayoud, superior metaxenia traits in males, high yielding, etc.); (3) creation of uniform, spirited, and disease-free plants; (4) no impact of the season on plant productivity; (5) enables the transfer of plant materials (Muirhead 1961). It is pollinated by Pinu. sylvestris in India and Pakistan and by Phoenix canariensis in Spain (Oudejans 1979; Benbades 1992). This species now has a huge amount of genetic variation as a result of this significantly outbreeding habit. According to Zaid and de Wet (1999), there are 3,000 varieties in existence worldwide. According to Bashah (1996), around 450 varieties of dates are grown in Saudi Arabia, 400 in Iran, 600 in Iraq, 250 in Tunisia, 244 in Morocco, and many more in other nations.
One of the most modern techniques used for date palm (Phoenix dactylifera L.) multiplication and manufacture of disease-free offshoots is tissue culture, cell suspension culture, and artificial seeds. Additionally, it allows for the multiplication of date palms, which was previously impossible. Numerous variables have a significant impact on date palm micropropagation (El-Kosary 2004). By adopting high-quality planting materials from excellent genetic stock, date palm yield and productivity may be greatly increased. In vitro clonal propagation is a potent and effective replacement for traditional vegetative propagation for ensuring rapid multiplication and establishment of true-to-type plants of superior cultivars (Al-Khayri 2005, 2007). The requirement for planting materials for date palms is estimated to require seven million plants annually. Procedures for date palm tissue-culture propagation have been created. Techniques using tissue culture were successful in commercially propagating date palms (Hoop 2000).
Since breeding and tissue culture alone cannot create new excellent plant cultivars, genetic transformation offers an interesting new tool to enhance conventional crop improvement techniques. Together, these methods should hasten the generation of new superior plant varieties (Hassan 2013). Recombinant DNA technology, which enables the introduction of a single new characteristic without changing the cultivar’s current traits, holds enormous promise for crop enhancement applications (Hansen and Wright 1999; Ahmed et al. 2000).
Thionin show a broad cellular toxicity against wide range of organisms and eukaryotic cell lines; while possessing some selectivity. By directly interacting with the membrane, thionins are thought to play a role in defense against bacteria, fungi, and other plant pathogens (Stec 2006; Hussien 2020).
Manipulation of chitosan nanoparticles in thionin genetic transformation into plant tissues was applied in this study. By producing antifungal Thionin proteins, we aimed to give date palm plants resistance to fungi in this work (Thio-60). Using chitosan nanoparticles, the thionin gene was introduced into the tissues of the date palm. After confirming that the thionin gene can be transformed into transgenic date palm cultivars, the fungal infection was examined for resistance of transgenic plants against Fusarium oxysporum. Abdel-Razik et al. (2017), Hussien (2020) and Tawfik et al. (2022) has approved that thionin genes has antifungal activities against several fungal and bacterial infections via using chitosan nanoparticles as carrier for genes. They also have approved that chitosan nanoparticles are efficient in genetic transformation into different plant tissues such as potato, Paulownia and onion, respectively.
Chitosan nanoparticles are applied for gene transformation for the following reasons: cheap, biodegradable, biocompatible, nontoxic and save time compared to traditional Agrobacterium method. In Agrobacterium transformation, it requires co-cultivation, pre-selections for 3–5 days and requires antibiotics like cefotaxime to inhibit the action of Agrobacterium. Otherwise, using chitosan in transformation is faster as it only requires about half an hour to transform the plasmid into plant tissues, and it doesn’t require antibiotics. Also, chitosan nanoparticle transformation efficiency is higher than that of Agrobacterium transformation (Hussien 2020; Tawfik et al. 2022).
Conclusion
Acclimatization of plant tissue culture conditions was approved for the three date’s cultivars, including media constituent’s and hormone concentrations. Thionin gene was translated into thionin protein which has antimicrobial activity against phytopathogenic fungi. Genetic transformation was performed by chitosan nanoparticles, and the gene was transferred into 3 date’s cultivars (Barhy, Sakkoti, and Shamia). Conventional PCR was applied to confirm genetic transformation into date palm. The thio-60 gene was successfully transferred into the three date’s cultivars. After confirming that the thionin gene can be transferred into transgenic date palm cultivars, the fungal infection with Fusarium oxysporum was applied to estimate the resistance of the transgenic cultivar lines. The transgenic lines of date’s cultivars showed higher resistance and decrease in the inhibition percentage resulted from fungal infection.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Abdel-Razik AB, Hammad IA, Tawfik E (2017) Transformation of thionin genes using chitosan nanoparticle into potato plant to be resistant to fungal infection. IOSR-JBB 3:1–13
Abdelmonem AM, Rasmy MR (2007) Major diseases of date palm and their control. Commun Inst For Bohem 23:9–23
Ahmed KZ, Allam HZ, Sági F (2000) Cereal genetic engineering: prospects and limitations. Egypt J Genet Cytol 29(1):103–126
Al-Khalifah NS, Askari E, Shanavas Khan AE (2012) Molecular and morphological identification of some elite varieties of date palms grown in Saudi Arabia, Emirates. J Food Agric 24:456–461
Al-Khayri JM (2005) Date palm Phoenix dactylifera L. Protocol for somatic embryogenesis in Woody plants. Springer, Dordrecht, pp 309–319
Al-Khayri JM (2007) Micropropagation of date palm Phoenix dactylifera L. In: Jain SM, Haggman H (eds) Protocols for Micropropagation of Woody Trees and Fruits. Springer, Berlin, pp 509–526. https://doi.org/10.1007/978-1-4020-6352-7_46
Bashah MA (1996) Date Variety in the Kingdom of Saudi Arabia, Guidance booklet: palms and dates. King Abdulaziz University Press, Riyadh, Saudi Arabia, pp 1225–1319
Benbades AK (1992) Coconut and Date palm, In: Benbades, A. K. and F. E. Hammerschlag (eds.), Biotechnology of Perennial Fruit Crops, 383–400
El-Kosary S (2004) 1–67. Recent techniques in propagation of date palm. Date Palm Micropropagation. https://doi.org/10.13140/RG.2.1.1752.2082
George EF, Hall MA, Greet-Jan DK (2008) Plant tissue culture procedure-background. Plant propagation by tissue culture. Springer, Dordrecht, pp 1–28
Hansen G, Wright MS (1999) Recent advances in the transformation of plants. Trends Plant Sci 4:226–231. https://doi.org/10.1016/s1360-1385(99)01412-0
Hassan L (2013) Successful genetic transformation in date palm (Phoenix dactylifera). J Bangladesh Agricultural Univ 11:171–176. https://doi.org/10.3329/jbau.v11i2.19841
Hoop BMD (2000) Date palm micropropagation in Saudi Arabia: policies and technology transfer. Int J Biotechnol 2:333–341. https://doi.org/10.1504/IJBT.2000.000143
Hussien ET (2020) Production of transgenic Paulownia tomentosa (Thunb.) Steud- using chitosan nanoparticles to express antimicrobial genes resistant to bacterial infection. Mol Biol Res Commun 9:55–62. https://doi.org/10.22099/mbrc.2019.35331.1454
Johnson DV (2011) Introduction: date palm biotechnology from theory to practice. Date Palm Biotechnology. Springer, Dordrecht, pp 1–11
Khan RS, Sjahril R, Nakamura I, Mii M (2008) Production of transgenic potato exhibiting enhanced resistance to fungal infections and herbicide applications. Plant Biotechnol Rep 2:13–20. https://doi.org/10.1007/s11816-008-0043-x
Khokhar MI, Teixeira da Silva JA (2017) Date palm (Phoenix dactylifera L.) biotechnology: a mini review, BioTechnologia. J Biotechnol Comput Biology Bionanotechnology 98. https://doi.org/10.5114/bta.2017.68315
Li Guang-feng, Wang Jing-cheng, Feng Xin-min, Liu Zhen-dong, Jiang Chao-yong (2015) Preparation and testing of quaternized chitosan nanoparticles as gene delivery vehicles. Appl Biochem Biotechnol 175:3244–3257. https://doi.org/10.1007/s12010-015-1483-8
Maniatis T, Sambrook J, Fritsch ER (1982) Molecular cloning: a Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory, New York, U.S.A.
Mansoori B, Kord MH (2006) Yellow death: a disease of date palm in Iran caused by Fusarium solani. J Phytopathol 154:125–127. https://doi.org/10.1111/j.1439-0434.2006.01067.x
Muirhead D (1961) Palms,Dale Stuart King Publishers, Arizona
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Oudejans JHM (1979) Date palm (Phoenix dactylifera, Palmae). In: Simmonds NW (ed) Evolution of crop plants. Longman, London
Peppas NA, Huang Y (2004) Nanoscale technology of mucoadhesive interactions. Adv Drug Deliv Rev 56:1675–1687. https://doi.org/10.1016/j.addr.2004.03.001
Pourhosseini L, Kermani MJ, Habashi AA, Khalighi A (2013) Efficiency of direct and indirect shoot organogenesis in different genotypes of Rosa hybrida. Planr Cell Tissue Organ Cult 112:101–108. https://doi.org/10.1007/s11240-012-0210-1
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76. https://doi.org/10.1007/BF00020088
Shuaib M, Zeb A, Ali Z, Ali W, Ahmed T, Khan I (2007) Characterization of wheat varieties by seed storage protein electrophoresis. Afr J Biotechnol 6:497–500. https://doi.org/10.4314/ajb.v6i5.56863
Stec B (2006) Plant thionins–the structural perspective. Cell Mol Life Sci. 63(12):1370-85. https://doi.org/10.1007/s00018-005-5574-5. PMID: 16715411
Tawfik E, Ahmed MF (2022) Chitosan nanoparticles as a new technique in gene transformation into different plants tissues. Nat Resour Hum Health 1–7. https://doi.org/10.53365/nrfhh/144414
Tawfik E, Hammad I, Bakry A (2022) Production of transgenic Allium cepa by nanoparticles to resist aspergillus niger infection. Mol Biol Rep 49:1783–1790. https://doi.org/10.1007/s11033-021-06988-5
Zaid A, Arias-Jimenez EJ (2002) Date palm cultivation. FAO Plant production and protection paper 156, Rev. 1. FAO, Rome
Zaid A, De Wet PF (1999) Chapter I Botanical and Systematic Description of Date Palm. FAO Plant Production and Protection Papers 1–28
Acknowledgements
The authors would like to thank Agricultural Center for Genetic Engineering and Biotechnology “ACGEB”, Faculty of Agriculture, Ain Shams University for facilitation of working in tissue culture labs. Also, we would like to thank Botany and Microbiology Department, Faculty of Science, Helwan University.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Material, E.G.H.A, E.T.H, A.B.A., conceptualization, E.G.H.A, K.Z.A, A.B.A, methodology, K.W.A, E.T.H; formal analysis, K.W.A, E.T.H.; writing original draft preparation, K.W.A.; E.T.H; writing-review and editing, E.G.H.A, K.Z.A, E.T.H., A.B.A.; visualization, E.G.H.A, K.Z.A, A.B.A.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Communicated by Melekşen Akın.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allah, K.W.A., Alabasey, E.E.D.G.H., Ahmed, K.Z. et al. Phoenix dactylifera in vitro culture and transformation of Thio-60 antifungal gene via chitosan nanoparticle. Plant Cell Tiss Organ Cult 155, 603–612 (2023). https://doi.org/10.1007/s11240-023-02505-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02505-7