Abstract
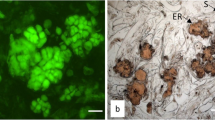
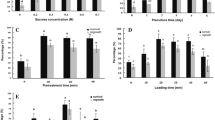
The goal of the study was to quantify the effect of different cryoprotective pretreatments on the maturation ability of cryopreserved Pinus radiata embryogenic tissue. Fourteen cell lines were evaluated under five pretreatments: (1) Dimethyl sulfoxide 5% (v/v); (2) Dimethyl sulfoxide 10% (v/v); (3) Dimethyl sulfoxide 5% (v/v) and 0.09 M of l-proline; (4) Dimethyl sulfoxide 10% (v/v) and 0.09 M of l-proline; (5) 0.09 M of l-proline and compared to embryogenic tissue that was not cryopreserved (control). The cell lines were thawed at 37 °C to evaluate the recovery percentage and determine the maturation ability. The recovery rate of all cryopreserved material was significantly lower than the control and material treated with 0.09 M of l-proline, in absent dimethyl sulfoxide, showed the lowest levels of recovery. Regarding the number of embryos, an interaction between pretreatments and the cell lines under evaluation was identified. Moreover, morphological differences in the plants being treated with the most concentrated Dimethyl sulfoxide pretreatment was observed. At the microscopic level, no damage or morphological changes in proembryogenic masses were observed during the post-cryopreservation evaluation. Therefore, the best pretreatment was 5% Dimethyl sulfoxide supplemented with 0.09 M l-proline, allowing the most growth recovery of thawed samples and more cotyledonary embryos (maturation ability). The evaluated responses in presence of cryoprotective elements were genotype dependent.





Similar content being viewed by others
Abbreviations
- ET:
-
Embryogenic tissue
- Se:
-
Somatic embryo
- DMSO:
-
Dimethyl sulfoxide
- ECL:
-
Embryogenic cell line
- ECLs:
-
Embryogenic cell lines
- PEM:
-
Proembryogenic mass
- PEMs:
-
Proembryogenic masses
References
Álvarez JM, Cortizo M, Ordás RJ (2012) Cryopreservation of somatic embryogenic cultures of Pinus pinaster: effects on regrowth and embryo maturation. Cryo Lett 33:476–484
Aronen T, Krajnakova J, Häggman H, Ryynänen L (1999) Genetic fidelity of cryopreserved embryogenic cultures of open-pollinated Abies cephalonica. Plant Sci 142:163–172
Benson E (2008) Cryopreservation theory. In: Reed B (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 15–32
Burrit D (2012) Proline and the cryopreservation of plant tissue: Functions and practical aplications. In: Katkov I (ed) Current frontiers in cryopreservation. Intech, Croatia, pp 415–430
Carneros E, Hernández I, Toribio M, Díaz-Sala C, Celestino C (2017) Effect of different cryoprotectant procedures on the recovery and maturation ability of cryopreserved Pinus pinea embryogenic lines of different ages. In Vitro Cell Dev Biol Plant 53:469–477
DeVerno L, Park Y, Bonga J, Barret J (1999) Somaclonal variation in cryopreserved embryogenic clones of white spruce (Picea glauca (Moench) Voss.). Plant Cell Rep 18:948–953
Filonova L, Bozhkov P, Von Arnold S (2000) Development pathway of somatic embryogenesis in Picea abies as revelead by time-lapse tracking. J Exp Bot 51:249–264
Ford C, Jones N, Van Staden J (2000) Optimization of a working cryopreservation protocol for Pinus patula embryogenic tissue. In Vitro Cell Dev Biol Plant 36:366–369
Fraga H, Vieira L, Puttkammer C, Da Silva J, Don Anjos K, Oliveira E, Guerra M (2016) High-efficiency cryopreservation of Araucaria angustifolia (Bertol.) Kuntze embryogenic cultures: ultrastructural characterization and morpho-physiological features. Plant Cell Tissue Organ Cult 124:307–318
García-Mendiguren O, Montalbán I, Goicoa T, Ugarte M, Moncaleán P (2015) Environmental conditions at the initial stages of Pinus radiata somatic embryogenesis affect the production of somatic embryos. Trees 30:949–958
Gupta P, Durzan D, Finkle B (1987) Somatic polyembryogenesis in embryogenic cell masses of Picea abies (Norway spruce) and Pinus taeda (Loblolly pine) after thawing from liquid nitrogen. Can J For Res 17:1130–1134
Häggman H, Ryynänen L, Aronen T, Krajnakova J (1998) Cryopreservation of embryogenic cultures of Scots pine. Plant Cell Tissue Organ Cult 54:45–53
Häggman H, Aronen T, Ryynänen L (2000) Cryopreservation of embryogenic cultures of conifers. In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis and woody plants. Public Academic Publishers, Dordrecht, pp 707–727
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. Cryo Lett 25:3–22
Hargreaves C, Smith D (1992) Cryopreservation of Pinus radiata embryogenic tissue. Int Plant Prop Soc Comb Proc. 42:327–333
Hargreaves C, Grace L, Holden D (2002) Nurse culture for efficient recovery of cryopreserved Pinus radiata D. Don embryogenic cell lines. Plant Cell Rep 21:40–45
Hazubska-Przybyl T, Dering M (2017) Somaclonal variation during Picea abies and P. omorika somatic embryiogenesis and cryopreservation. Acta Biol Crac Ser Bot 59:93–103
Kartha K, Fowke L, Leung N, Caswell K, Hakman I (1988) Induction of somatic embryos and plantlets from cryopreserved cell cultures of white spruce (Picea glauca). J Plant Physiol 132:529–539
Kristensen M, Find J, Floto F, Moller J, Norgaard J, Krogstrup P (1994) The origin and development of somatic embryos following cryopreservation of an embryogenic suspension culture of Picea sitchensis. Protoplasma 182:65–70
Lainé E, Bade P, David A (1992) Recovery of plants from cryopreserved embryogenic cell-suspensions of Pinus caribaea. Plant Cell Rep 11:295–298
Lambardi M, Ozudogru E, Barberini S, Danti R (2018) Strategies for fast multiplication and conservation of forest trees by somatic embryogenesis and cryopreservation: a case study with cypress (Cupressus sempervirens L.). Not Bot Horti Agrobot 46(1):32–38
Latutrie M, Aronen T (2013) Long-term cryopreservation of embryogenic Pinus sylvestris cultures. Scand J For Res 28(2):103–109
Litvay J, Verma D, Johnson M (1985) Influence of a loblolly pine (Pinus taeda L.). Culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep 4:325–328
Martínez-Montero M, Harding K (2015) Cryobionomics: evaluating the concept in plant cryopreservation. In: Barh D, Sarwar M (eds) PlantOmics: the omics of plant science. Springer, New Delhi, pp 655–682
Marum L, Estevao C, Oliveira M, Amancio S, Rodrígues L, Miguel C (2004) Recovery of cryopreserved embryogenic cultures of maritime pine-effect of cryoprotectant and suspensión density. Cryo Lett 25:363–374
Mathur G, Alkutkar V, Nadgauda R (2003) Cryopreservation of embryogenic culture of Pinus roxburghii. Biol Plant 46:205–210
Montalbán I, Moncaleán P (2017) Long term conservation at -80 °C of Pinus radiata embryogenic cell lines: recovery maturation and germination. Cryo Lett 38(3):202–209
Nörgaard J, Duran V, Johnsen O, Krogstrup P, Baldursson S, Von Arnold S (1993) Variation in cryotolerance of embryogenic Picea abies cell lines and the association to genetic, morphological, and physiological factors. Can J For Res 23:2560–2567
Nunes S, Marum L, Farinha N, Tolentino V, Almeida T, Dias M, Santos C (2017) Plant regeneration from ploidy-stable cryopreserved embryogenic lines of the hybrid Pinus elliottii x P. caribaea. Ind Crops Prod 105:215–224
Ogawa Y, Sakurai N, Oikawa A, Kai K, Morishita Y, Mori K, Moriya K, Fujii F, Aoki K, Suzuki H, Ohta D, Kazuki S, Shibata D (2012) High-throughput cryopreservation of plant cell cultures for functional genomics. Plant Cell Physiol 53(5):943–952
Ozudogru E, Lambardi M (2016) Cryotechniques for the long-term conservation of embryogenic cultures from woody plants. In: Germana M, Lambardi M (eds) In vitro embryogenesis in higher plants, methods in molecular biology. Springer, New York, pp 537–550
Panis B, Lambardi M (2005) Status of cryopreservation technologies in plants (crops and forest trees). In: The role of biotechnology for the characterization and conservation of crop, forest, animal and fishery genetic resources in developing countries. FAO, Turin, pp 43–54
Pullman G, Johnson S, Peter G, Cairney J, Xu N (2003) Improving loblolly pine somatic embryo maturation: comparison of somatic and zygotic embryo morphology, germination, and gene expression. Plant Cell Rep 21:747–758
Salaj T, Matusikova I, Fraterova L, Pirselova B, Salaj J (2011) Regrowth of embryogenic tissues of Pinus nigra following cryopreservation. Plant Cell Tissue Organ Cult 106:55–61
Salaj T, Matusikova I, Swennen R, Panis B, Salaj J (2012) Long-term maintenance of Pinus nigra embryogenic cultures through cryopreservation. Acta Physiol Plant 34:227–233
Salaj T, Matusova R, Swennen R, Panis B, Salaj J (2016) Tissue regeneration of Abies embryogenic cell lines after 1 year storage in liquid nitrogen. Biologia 71(1):93–99
Schumacher H, Westphal M, Heine-Dobbernack E (2015) Cryopreservation of plant cell lines. In: Cryopreservation and freeze-drying protocols: methods in molecular biology. Springer, New York, pp 423–429
Toivonen P, Kartha K (1989) Cryopreservation of cotyledons of nongerminated white spruce (Picea glauca (Moench) Voss) embryos and subsequent plant regeneration. J Plant Physiol 134(6):766–768
Touchell D, Chiang V, Tsai C (2002) Cryopreservation of embryogenic cultures of Picea mariana (black spruce) using vitrification. Plant Cell Rep 21:118–124
Varis S, Ahola S, Jaakola L, Aronen T (2017) Reliable and practical methods for cryopreservation of embryogenic cultures and cold storage of somatic embryos of Norway spruce. Cryobiology 76:8–17
Von Arnold S, Larsson E, Panagiotis N, Moschou P, Zhu T, Uddenberg D, Bozhkov P (2016) Norway spruce as a model for studying regulation of somatic embryo development in conifers. In: Park Y, Bonga J, Moon H (eds) Vegetative propagation of forest trees. Korea Forest Research Institute, Seoul, pp 351–372
Vondrakova Z, Cvikrova M, Eliasova K, Martincova O, Vagner M (2010) Cryotolerance in Norway spruce and its association with growth rates, anatomical features and polyamines of embryogenic cultures. Tree Physiol 30:1335–1348
Walter C, Find J, Grace L (2005) Somatic embryogenesis and genetic transformation in Pinus radiata. In: Jain S, Gupta P (eds) Protocol for somatic embryogenesis in woody plants. Springer, Dordrecht, pp 11–24
Withers L, King P (1980) A simple freezing unit and routine cryopreservation method for plant cell cultures. Cryo Lett 1:213–220
Acknowledgements
This study was carried out with the financing and plant material from the Forest research company Bioforest S.A. The authors thanks Dr. Mauricio Ramírez for his contribution on the reviewing of the manuscript and his collaboration in the statistical analysis.
Author information
Authors and Affiliations
Contributions
YL, DR performed the planning and design of experiments. YL performed the research and wrote the manuscript. CB XM helped with the technical support and review the results and MS read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Communicated by M. Angeles Revilla.
Rights and permissions
About this article
Cite this article
Lineros, Y., Balocchi, C., Muñoz, X. et al. Cryopreservation of Pinus radiata embryogenic tissue: effects of cryoprotective pretreatments on maturation ability. Plant Cell Tiss Organ Cult 135, 357–366 (2018). https://doi.org/10.1007/s11240-018-1469-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1469-7