Abstract
As the world races towards a plant-based bioeconomy, plants known to be ideal and economical bioreactors are being harnessed for the production of recombinant proteins. The major immunodominant 10 kDa GroES TB antigen (Chaperonin 10) gene from Mycobacterium tuberculosis was selected for expression in plants as a putative tuberculosis (TB) subunit vaccine candidate. Two crops, tobacco and potato, were engineered by stable plant transformation for expression of the 10 kDa GroES TB antigen using non-viral binary vectors. The integration of the GroES TB gene into the genomes of tobacco and potato was confirmed by PCR and Southern blotting. The expression of the GroES TB antigen in tobacco was 0.04–1.2 % of the total soluble protein (TSP). However, the expression of the same TB antigen in the Indian potato cv. Kufri bahar was comparatively low (0.033 % of TSP). The recombinant GroES plant derived protein was characterised and confirmed by MALDI-TOF–TOF and ELISA. This is the first report of the expression of the 10 kDa chaperonin in tobacco and potato.
Similar content being viewed by others
Introduction
Tuberculosis is the second most infectious disease posing a global public health threat. Its control and management have been complicated by multi-drug resistance (Cohn et al. 1997) and latent infection, which have led to the search for new and more effective drugs as well as vaccine candidates. A third of the world’s population has contracted the disease and almost 2 million die every year (Dye et al. 1999; Bloom and Murray 1992; Chakhaiyar and Hasnain 2004). The HIV/TB combination is deadly and one speeds the progress of the other. A quarter of a million deaths among the HIV patients are due to TB and one of the effective means to save the HIV patients is to control TB (Dye et al. 2002; Siddiqi et al. 2002; Ahmed et al. 2003). The currently used Bacille Calmette-Guérin (BCG), an attenuated vaccine derived from Mycobacterium bovis, has been found to have variable efficacy in TB control (Fine 1989; Orme et al. 2001).
Mycobacterium tuberculosis is the pathogen that causes the disease in man and the primary site of infection is the respiratory tract. An effective vaccination strategy may involve an induction of immunity at these sites that would interfere with pathogen colonisation and invasion before infection is established leading to the disease. This is achieved most effectively by local delivery of the vaccine, since administration of vaccines by injection generally stimulates poor mucosal immune responses. The most convenient means to achieve this is via the oral or nasal route. Therefore, a mucosal vaccination should have an inherent advantage in producing a protective immune response over the traditional parenteral immunization.
In the last decade there has been a shift from traditional vaccines to subunit vaccines owing to some of the reaction problems associated with them. The subunit vaccine comprises only of those factors against which a protective immune response would ideally be elicited, while other factors responsible for side effects are eliminated. The subunits may be peptide or protein antigens, polysaccharides or DNA. Development of subunit vaccines for TB, though in preliminary stages, holds promise. The ESAT 6 (6 kDa early secretory antigenic target) of M. tuberculosis has been expressed in plants (Rigano et al. 2004; Zelada et al. 2006). The LTB-ESAT-6 fusion elicited the antigen-specific responses from CD4+ cells, interferon-γ (IFN-γ) production, and CD8+ proliferation equivalent to BCG, but the IFN-γ production failed to reduce the bacterial load and protect the mice on challenge. Dorokhov et al. (2007) have expressed high levels of the ESAT6 and Ag85B antigens from M. tuberculosis in tobacco, individually as well as a fusion product.
Steyn et al. (2003) showed that M. tuberculosis genes of LprF and LprJ ligand-binding proteins were expressed in yeast. Piubelli et al. (2013) showed that TB10.4, Ag85B and a TB10.4-Ag85B chimeric protein immunodominant antigens of M. tuberculosis were expressed in Escherichia coli and purified to homogeneity. Recombinant Ag85B antigen was expressed in two E. coli strains (JM109DE3 and Origami B) (Lu et al. 2007). Dhar et al. (2004) used recombinant BCG strains overexpressing Ag 85A and Ag 85C, to immunize BALB/c mice with recombinant BCG strains which exhibited an increased humoral immune response when compared to mice immunized with wild-type BCG, but its immunogenic potential is yet to be reported. In the absence of a universally accepted vaccine, development of an effective vaccine is the holy grail of TB research. The identification of the right target and engineering high levels of protein singly or as a fusion product that would elicit the desired immune responses are the first steps in this direction.
In this study, we have selected the major immunodominant 10 kDa GroES TB antigen (Chaperonin 10) as a putative candidate for a TB subunit vaccine development in plants. The Chaperonin 10 gene was first isolated from M. tuberculosis H37Rv by Baird et al. (1989); it encodes 99 amino acids with a molecular mass of 10.7 kDa. The study by Barnes et al. (1992), suggested that the 10 kDa TB antigen is a highly immunoreactive antigen and should be evaluated for its capacity to elicit protective immunity. Mattow et al. (2003) and Bellinzoni and Riccardi (2003) have expressed that the 10 kDa chaperonin is one of the candidate antigens for novel vaccine production. The present study was undertaken with a view to express the 10 kDa GroES TB antigen in tobacco and potato. The long term objective of this study was to express it primarily in tobacco, for testing the plant produced antigen for its parenteral immune response, and secondarily in potato, to test its mucosal immune response for developing edible vaccines.
Materials and methods
Construction of the binary vector
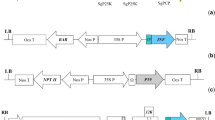
The GroES TB antigen gene was isolated from the pCVA5 vector (Arvindhan 2005) by PCR as an NcoI–XbaI fragment (330 bp). The digested TB antigen gene was subcloned as an NcoI–XbaI fragment into pVHGT103 resulting in pSSKvG301. The pSSKvG301 construct had the CaMV 35 S promoter with the Omega factor flanked by the Kozak sequence abutting the NcoI site at its 3′ end, driving the TB antigen gene which lies adjacent. The CaMV poly A terminator was positioned at the 3′ end of the gene. The clone pSSKvG301 was digested with HindIII that released the TB antigen gene cassette (1.2 kb). The TB antigen gene cassette was subcloned into the HindIII site in the multi-cloning site (MCS) of the binary vector pCAMBIA 2300 and pCAMBIA 1304 (Fig. 1). PCR positive clones were named as pSSKvA302 and pTBSJ2 respectively after confirmation by sequencing of the gene cassette using primer 23 TB F and R (F-5′ GATCCTCTAGAGTCGACCTGC 3′ and R-5′ TCCCAGTCACGACGTTGTA 3′) and restriction digestion. The two binary vectors were mobilized into Agrobacterium tumefaciens LBA 4404 for plant transformation.
Growth and maintenance of Tobacco plants
Tobacco, Nicotiana tabacum cv. Petit havana seeds were sterilised in a microfuge tube under aseptic conditions in 70 % alcohol for 1 min followed by 0.1 % mercuric chloride for 8 min. The seeds were washed well in sterile water, briefly dried before being plated on N. tabacum medium (NTM) medium (50 % Murashige and Skoog (MS) basal medium (Murashige and Skoog 1962), MS vitamins, 2 % Sucrose, pH 5.8, 0.8 % agar). It was in the light at 26 ± 2 °C with 16 h photoperiod. The in vitro grown plants were subcultured and maintained on NTM medium using single nodes and shoot tips as explants. Plants were acclimatised prior to transfer to the greenhouse by transferring well rooted plants to pots containing sterile potting mix (red soil: sand: farm, yard, manure, 1:1:1) and maintained in the greenhouse.
Growth and maintenance of potato plants
In vitro cultures of potato, Solanum tuberosum L., variety Kufri bahar, were procured from Central Potato Research Institute, Shimla, India. The cultures were maintained by single node cuttings on S. tuberosum node (STN) medium (MS basal. O.4 mg/l Thiamine HCl, 2.0 mg/l calcium pantothenic acid, 0.25 mg/l GA3, 100 mg/l inositol, 3 % sucrose, 0.8 % agar, pH 5.8). Plants were subcultured onto fresh medium once in 4 weeks. The cultures were maintained in the light at 24 ± 2 °C and 16 h photoperiod.
In vitro tuber induction
Stem segments of about five nodes each trimmed of their leaves were grown in 250 ml conical flasks in S. tuberosum stem (STS) liquid medium (MS basal medium, 0.4 mg/l Thiamine HCl, 2.0 mg/l Calcium Pantothenic acid, 0.4 mg/l GA3, 0.5 mg/l Benzyladenine (BA), 0.01 mg/l NAA, 100 mg/l Inositol, 2 % Sucrose, pH 5.8). The cultures were incubated on a shaker at 100 rpm, under 3,000 lux and a photoperiod of 16 h light and 8 h dark at 24 ± 2 °C. After 3 weeks the STS medium was replaced with S. tuberosum transformation (STT) liquid medium (MS basal medium, 0.4 mg/l Thiamine HCl, 5.0 mg/l BA, 0.01 mg/l NAA, 1,000 mg/l Inositol, 8 % Sucrose, pH 5.8) for tuber induction. It was in the dark for 9–10 weeks at 18 ± 2 °C. The tubers were harvested and stored in sterile Petri plates at 4 °C.
Stable transformation of tobacco leaf using Agrobacterium
Nicotiana tabacum cv. Petit havana leaf explants of 1 cm2 were pre-incubated on MS medium supplemented with BA 1 mg/l and Naphthalene acetic acid (NAA) at 0.1 mg/l [N. tabacum regeneration (NTR)]. After 3 days, the leaf discs were infected with the Agrobacterium strain LBA4404 carrying the binary construct. Both pSSKv302 and pTBSJ2 binary constructs were used for tobacco transformation. The agrobacterial cells were grown in AB minimal liquid media with 20 µM acetosyringone with the appropriate antibiotics and grown overnight until an OD of 0.8 was reached at 600 nm. This culture was re-suspended in MS medium containing 100 µM acetosyringone and was used for infecting the leaf explants. The leaf explants were co-cultured on filter discs moistened with MS medium amended with 100 µM acetosyringone placed on NTR agar plates for 3 days in the dark at 26 ± 2 °C. The leaf explants were then placed on NTR medium amended with the appropriate antibiotics and it was in the light. The elongated regenerated shoots were transferred to NTM medium with antibiotics until they rooted. The rooted plants were acclimatized and maintained in the greenhouse.
Transformation of potato internodes using Agrobacterium
Internodes were explanted from 4 week old potato cv. Kufri bahar cultures pre-cultured in Petri plates on S. tuberosum internodes (STI) medium (MS basal medium, 0.4 mg/l Thiamine HCL, 0.5 mg/l Nicotinic acid, 0.5 mg/l Pyridoxine HCl, 2.0 mg/l Calcium Pantothenic acid, 2.0 mg/l GA3, 0.75 mg/l Zeatin, 100 mg/l Inositol, 3.0 % Sucrose, 0.3 % Phytagel, pH 5.8). The cultures were incubated in a 16 h photoperiod regime at 24 ± 1 °C. After 3 days, the explants were infected with Agrobacterium strain LBA 4404 carrying the binary construct pTBSJ2 for 10 min. The bacterial culture preparation was as described in the earlier section. The explants were briefly blotted dry and co-cultured on filter discs (9 cm diameter) moistened with MS medium amended with 100 µM acetosyringone placed on STI agar plates. After 3 days in the dark at 24 ± 2 °C, the internodes were subcultured on STI medium amended with the antibiotic Hyg (15 mg/l) and it was in the light. The elongating shoots were periodically subcultured onto STN medium with Hyg (20 mg/l) until they rooted.
PCR amplification of the transgene from genomic DNA of T0 progeny
Genomic DNA was isolated from leaves of T0 progeny plants as described by Berendzen et al. (2005). The presence of GroES TB antigen gene was determined by PCR analysis using the primers TBSJ1 F (5′ CGGGGTACCTATTTTTACAACAATTACCAACAAC 3′) and TBSJ1R (5′ CGAGCTCGGAACACGCTCTACTACTTG 3′) (400 bp). With genomic DNA as the template the TB antigen gene was amplified with thermocycling conditions as follows: 94 °C for 5 min, 30 cycles of 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min and 7 min final extension at 72 °C. The PCR products were analysed on a 1 % agarose gel. Similarly the potato T0 progenies were analysed by PCR for both the TB antigen gene as above and the antibiotic selection marker, hygromycin phoshotransferase, using the Hpt F (5′ GACGATTGCGTCGCATCGACC 3′) and R primer (5′ CAGCGTCTCCGACCTGCTGCA 3′) (1 kp) under the above mentioned thermocycling conditions.
Southern hybridisation
Genomic DNA was isolated from leaves of tobacco plants as described by Doyle and Doyle (1990). For the Southern analysis, 15 µg genomic DNA from untransformed and the transgenic plants from both lines was digested with restriction enzyme HindIII, electrophoresed through a 1.2 % agarose gel and blotted onto a Hybond N nylon membrane (Amersham Biosciences, USA) using a vacuum blotter. Hybridization was done following standard procedures (Sambrook et al. 1989) using the 32P-labelled TB antigen gene (~400 bp) as well as a CaMV 35S promoter (~600 bp) as probes to determine the TB antigen gene integration and its copy number, respectively in the transgenics.
Extraction of total soluble protein (TSP) from transgenic plants
The TSP was isolated from control and transgenics. The plant material was homogenized in a mortar with a pestle using liquid nitrogen and suspended in extraction buffer [100 mM Tris–Cl (pH 7.5), 1.0 mM EDTA, 2.0 mM PMSF, 10 % glycerol in the ratio of 1:1 (w/v)] and maintained on ice. Once the mixture had thawed it was centrifuged at 10,000 rpm at 4 °C for 15 min. The supernatant was transferred to a fresh microfuge tube. The extract was centrifuged repeatedly until a clear supernatant was obtained and stored at −20 °C for further use. The TSP extracted from leaf samples was determined by the Bradford method (Bradford 1976) using bovine serum albumin (Hi-media, India) as a standard.
Detection of rGroES by Western blot analysis
Fixed amounts of total protein extracts were boiled for 5 min in 5× SDS sample buffer and separated on 15 % Tris Tricine SDS-PAGE at constant 100 V. The electrophoresed protein was electro blotted onto the polyvinylidene fluoride (PVDF) membrane, blocked for 1 h (5 % skimmed milk) and labelled with the primary monoclonal antibody SA-12 overnight at 4 °C. The monoclonal GroES antibody, SA-12 (Colorado State University), was diluted in 15 ml PBST at a dilution of 1:50. After 3 washes in PBST the membrane was labelled with the secondary antibody for 3 h at room temperature. The secondary anti-mouse IgG raised in sheep conjugated to Horse radish peroxidase (HRP) enzyme (GE Healthcare Amersham, UK) was diluted in 2.5 % skimmed milk prepared in PBST at a dilution of 1:1,000. After three washes in PBST, the HRP activity was detected using the Enhanced Chemiluminesence ECL kit (Biological Industries, Israel) and was captured on X-ray film.
MALDI-TOF TOF analysis
In-gel digestion
Sliced Coomasie stained gel bands were washed twice with 50 % Acetonitrile (ACN) in 50 mM ammonium bicarbonate (NH4HCO3) for 15 min at room temperature (RT). The gel pieces were dehydrated by incubating them with 100 % ACN for 5 min at RT. Proteins were reduced using 10 mM DTT and alkylated with 50 mM iodoacetamide (IAA), both in 100 mM NH4HCO3. The gel pieces were dehydrated with ACN as described above, then the gel was completely dried at room temperature in a centrifugal evaporator and rehydrated in 50 mM NH4HCO3 containing 0.02 mg/ml modified trypsin (Promega). Proteins were digested by trypsin for 16–20 h at 37 °C. Then, the tryptic peptides in the supernatant were transferred to a sterile centrifuge tube and gel pieces were eluted by incubating the gel pieces with 0.1 % Trifluoroacetic acid (TFA) and 50 % ACN in an Ultrasonic water bath for 10 min at RT. The supernatant containing tryptic peptides were collected by centrifugation at 12,000g for 10 min. Additional peptides were extracted from gel pieces by repetition of the elution. The supernatant was collected and added to the previous one. Finally, the gel pieces were dehydrated by incubating the gel pieces with 100 % ACN for 20 min at RT. The pooled samples were dried and the peptides were extracted by centrifugal evaporation to near dryness; the peptides were extracted for MALDI-TOF MS analysis (Use of heat and drying for an extended time was avoided).
MALDI-TOF MS
The tryptic peptides extracted from the gel slices were resuspended in 5 µl of 50 % acetonitrile and 0.1 % TFA sonicated in a water bath. The sample was spun down and 0.5 µl of the sample was mixed with an equal volume of alpha-cyano-4-hydroxycinnamic acid matrix (10 mg/ml in 50 % ACN in H2O and 0.1 % TFA). The sample (0.5 µl) was applied to the steel target plate (MTP 384, Bruker) and analysed by MALDI-TOF MS (Autoflex and Ultraflex, Bruker Daltonics, Germany) and MALDI with MS/MS was Ultraflex TOF–TOF spectrometer (Bruker Daltonics, Germany) at the Molecular Biophysics Unit, Indian Institute of Science, Bangalore. The obtained mass spectra were searched against the M. tuberculosis complex database using MASCOT (http://www.matrixscience.com). The search parameters were: 100 ppm tolerance as the maximum mass error, monoisotopic mass value and fixed modification of cysteine by carboxymethyl. A protein was regarded identified if the matched peptide mass fingerprint covered 30 % of the complete protein sequence.
Quantification of rGroES in transgenics by dot blot assay and ELISA
Immuno-dot blot assay
The PVDF membrane was dipped in methanol for 5 min and approximately 50 µg of protein was spotted on the PVDF membrane and air dried at 37 °C. The membrane was blocked for 1 h. The membrane was rinsed twice in PBST and then incubated at 4 °C overnight in the primary antibody, SA-12, at a dilution of 1:100. After three 10 min washes in PBST, the membrane was incubated at room temperature for 1 h in the secondary anti-mouse IgG antibody conjugated to HRP at a dilution of 1:1,000 in PBST with 2.5 % skimmed milk. Three 10 min washes in PBST were followed by two washes in PBS to remove traces of Tween 20. The freshly prepared Diamino benzidine (60 mg DAB in 50 ml PBS, 200 µl of 30 % CoCl2, 200 µl of H2O2) substrate was added to the membrane and it was in the dark with constant shaking for 5–10 min until the reddish brown coloration appeared and the reaction was arrested with addition of dd.H2O. The membrane was washed briefly in dd.H2O and then rinsed in PBS before being documented.
ELISA
The pure protein GroES TB antigen at five different concentrations and the TSP were diluted in sodium carbonate coating buffer (15 mM Na2CO3, 35 mM NaHCO3) at a concentration of 200 µg/ml. TSP samples from control and transgenic tobacco (T0 and T1) along with control and transgenic potato plant and tuber were coated on the polystyrene ELISA plate. The samples were coated in triplicate and three wells with just the buffer were treated as blank. The plate was incubated at 4 °C overnight and washed with PBST (137 mM NaCl, 2.7 mM KCl, 10.0 mM Na2HPO4, 2.0 mM KH2PO4, Tween 0.05 %) twice. The wells were blocked [200 µl of blocking buffer 5.0 % Skimmed milk powder in prepared in PBST (0.1 % Tween 20)] at 37 °C for 1 h. This was followed by three washes with PBST and incubation with 100 µl per well of the primary antibody SA-12 at a dilution of 1:200 prepared in PBST for 1 h at 37 °C. The plate was washed thrice with PBST. Then 50 µl of the secondary antibody, Antimouse IgG linked to horse radish peroxidase whole antibody from sheep, was added at a dilution of 1:1,000 in PBST with 2.5 % skimmed milk powder. The plate was incubated at 37 °C for 1 h. The plate was washed thrice with PBST. Tetra methyl benzidine (TMB) substrate (a mixture of 6 ml each of Solution A and B, BD Biosciences) was added at 100 µl per well and it was in the dark for 20 min for the blue color development. The reaction was stopped with 50 µl of 2 N sulphuric acid. The absorbance was read at 490 nm with a plate reading at 450 nm in a ELISA reader (Molecular Devices, Spectra Max 250, USA).
Results
Molecular analysis of transgenics
The GroES TB antigen gene isolated from M. tuberculosis was cloned with the omega factor (Ω) at the 5′ end of the gene driven by a CaMV promoter and a CaMV poly A. Agrobacterium-mediated transformation of N. tabacum cv Petit havana resulted in 29 and 12 putative transgenic lines with the binary construct pSSKv302 and pTBSJ2, respectively. Screening of the tobacco putative T0 transgenic lines was done using PCR to detect the presence of the TB antigen gene in the plant genome. Of the 29 putative tobacco transgenic lines of the pSSKv302 construct, all 29 lines were positive for TBSJ1 F and R primer amplified product of ~400 bp (Fig. 2). Of the twelve putative transgenic lines of the pTBSJ2 construct, eight were positive for the above mentioned 400 bp amplified product (Fig. 3a). Potato cv Kufri bahar transformed with the second binary construct pTBSJ2 resulted in a single transformation event. The single putative potato transgenic line gave positive results with primer TBSJ1F and R (400 bp) for the GroES gene and with the primer Hpt F and R (1 kb) for the antibiotic selection marker, hygromycin phoshotransferase (Fig. 3b, c).
PCR confirmation of TB antigen gene in 29 putative tobacco transgenic lines resulting from Agrobacterium transformation using the pSSKv302 construct. Upper panel Lane M. 1 kb DNA ladder; 1 plasmid pSSKv302; 2–10 TBSJ1 T1–T9; 11 negative control; 12 untransformed tobacco; 13–17 TBSJI T10–T14. Lower panel Lane M 1 kb DNA ladder; 1 plasmid pSSKv302; 2–16 TBSJ1 T15–T 29; 17 negative control
PCR analysis of tobacco and potato putative transgenic lines. a TBSJ2 tobacco lines amplified with TBSJ1TB antigen gene specific primer. Lane M 100 bp DNA ladder; 1 Negative control; 2 untransformed tobacco; 4–11 TBSJ2 2 T5–2 T16; 3, 12–14 unexpressed transgenic lines; 15 plasmid pTBSJ2 DNA. b, c The putative potato line amplified with TBSJ1 F and R and Hygromycin phoshotransferase gene specific primer, respectively: b Lane M 100 bp DNA ladder; P plasmid pTBSJ2 DNA; T transgenic potato, UT untransformed potato, NC negative control, c Lane M 1 kb DNA ladder; P Plasmid pTBSJ2 DNA; UT untransformed potato, T transgenic potato, NC negative control
Southern analysis of putative tobacco transgenic plants
A random sampling of the PCR positive putative tobacco transgenic lines from both constructs was done and the genomic DNA was digested with the HindIII enzyme. The GroES TB gene integration was confirmed on probing the blot with a 32P labelled 400 bp amplicon of the GroES TB antigen gene. All the DNA samples of the transgenic tobacco lines gave a 1.2 kb signal in the blot as the HindIII digestion released the TB antigen gene cassette in its entirety (Fig. 4a). The number of copies of the TB antigen gene was confirmed by using the same HindIII digested genomic DNA but was probed with the radiolabelled CaMV 35 S promoter (0.6 kb) released from pSSKv302 fragment as a HindIII/XhoI digest. Two signals, one at 1.2 kb and the other greater than 8 kb were seen on the blot (Fig. 4b), confirming the presence of two copies of the CaMV 35S promoter, one driving the TB antigen gene and the other driving the selection marker (nptII or Hpt gene depending on the construct). The tested T0 samples showed single gene integrations.
Southern analysis of HindIII digests of plasmid and genomic DNA of untransformed and transgenic tobacco lines for. a Integration of the TB antigen gene into the plant genome probed witth 0.4 kb TB antigen gene. b Determination of TB antigen gene copy number probed with CaMV 35S promoter fragment of ~0.6 kb. Lane 1 1 kb DNA ladder; 2 Plasmid pSSKv302; 3 untransformed tobacco; 4 TBSJ1 T6; 5 T8; 6 T10; 7 T26; 8 T27; 9–10 T28; 11 T29; 12 TBSJ2 2T6; 13 2P2; 14 2P3
Characterization of the GroES TB antigen
On a 15 % Tris Tricine polyacrylamide gel about 200 µg of the total soluble protein (TSP) protein of the control tobacco and four tobacco transgenic lines TBSJ1 T6, T10, T27 and TBSJ2 2P1 that were confirmed by PCR and Southern blotting were run along with the culture filtrate antigen (CFA) of M. tuberculosis as a positive control. An additional band of the expected ~10.7 kDa size was observed in three of transgenic protein samples (Fig. 5) when compared to the control plant protein profile. The expected band of 10.7 kDa was found to be absent in TBSJ1 T6 plant protein sample.
Transgenic tobacco total soluble protein profile on 15 % Tris Tricine SDS-PAGE. Lane M Blue Ranger Prestained protein molecular weight marker (Pierce, Perbio); CF culture filtrate of M. tuberculosis; U untransformed tobacco plant protein; T6, T10, T27, 2P1 total soluble protein of tobacco transgenic lines of TBSJ1 T6, T10, T27 and TBSJ2 2P1, respectively
Western blot analysis of tobacco and potato transgenics
The TSP protein (about 300 µg) from control tobacco, two transgenic tobacco plants, TBSJ1 T8 and T10, control potato, transgenic potato plant and transgenic potato tuber were run on a 15 % Tris Tricine gel. Two positive controls, the M. tuberculosis culture filtrate antigen (60 µg) and the pure GroES TB antigen (20 µg) were also included. (Fig. 6a) Mapping of the protein of interest was done by Western Blot analysis using the SA-12 monoclonal antibody (Colorado State University) for the GroES antigen from M. Tuberculosis. The autoradiogram of the Western blot (Fig. 6b) picked up signals of 10.7 kDa size in the lanes having the culture filtrate antigen, the pure Cpn 10 (GroES) protein and in the protein samples of the tobacco transgenic lines T8 and T10. This confirmed the recombinant GroES TB antigen gene expression in tobacco. The same was not detected in the transgenic potato plant or tuber protein samples at this concentration.
Expression of recombinant CPN10 in tansgenic Tobacco and potato plants. a Coomasie Brilliant blue stained Tris Tricine SDS-PAGE: b Western blot labeled with SA-12 antibody. Lane M Prestained SDS-PAGE Protein marker (Bio-Rad, broad range); CFA culture filtrate antigen; CPN10 M. tuberculosis 10 kDa Chaperonin (CPN10); UT untransformed tobacco plant TSP; T8 TBSJ1 T8 plant TSP; T10 TBSJ1 T10 plant TSP; PC Untransformed potato plant TSP; PT transgenic potato plant TSP; PTT transgenic potato tuber TSP: Arrows show the 10 kDa protein (a) and its binding to SA-12 antibody (b)
MALDI-TOF/TOF analysis
The 10.7 kDa putative recombinant GroES (rGroES) protein band of interest derived from the transgenic tobacco lines TBSJ1 T8 and T10 were carefully excised and subjected to the trypsin in-gel digestion prior to a MALDI-TOF/TOF analysis. The MALDI MS of the Tryptic digest gave spectra with the mass/charge (m/z) values for both protein samples from T8 and T10 transgenics. The data were subjected to an in silico analytical MASCOT search to obtain the Peptide mass fingerprint (PMF) (Table 1). The MALDI MS data of the TBSJ1 T10 derived putative rGroES protein on a MASCOT search showed a probability based Mowse score of 109. A score >74 is significant (p < 0.05). The protein identity search gave the best match with the Chaperonin 10 of the Mycobacterium sp. The rGroES protein had 97 % sequence coverage (97 of the 100 amino acids) with all 10 masses matching with the expected peptide masses of the chaperonin-10 protein of M. tuberculosis (Table 1). The MALDI MS/MS of the precursor tryptic fragments of 2,342.4, 1,524.0 and 1,776.6 Da (Fig. 7) confirmed the sequences for these precursor peptide fragments derived from the recombinant GroES protein of both the transgenic lines. The MALDI MS/MS data aided the partial microsequencing of the three peptides mentioned above for both transgenics supporting the MALDI-MS derived PMF for the rGroES protein obtained from the same transgenic lines.
Immuno-dot blot assay in Tobacco (T0 and T1) and potato
As seen in Fig. 8, the Dot Blot assay showed positive reactions for the CFA, the T0 samples TBSJ1 T8 and T10, T1 progeny 8P1, 8P2, 8P3, 10P2 and 10P3 protein samples. The transgenic potato plant and transgenic potato tuber protein samples also gave positive reactions but not as intense as the CFA. However, the control tobacco, TBSJ1 T26 and the control potato showed no reaction.
Dot blot assay of the tobacco (T0 and T1 progeny) and potato transgenics. UT untransformed tobacco; T8 TBSJ1 T8 (T0); T10 TBSJ1 T10 (T0); T26 TBSJ1 T26 (T0); 8P1 TBSJ1 T8 (T1, seedling 1); 8P2 TBSJ1 T8 (T1, seedling 2); 8P3 TBSJ1 T8 (T1, seedling 3); 10P1 TBSJ1 T10 (T1, seedling 1); 10P2 TBSJ1 T10 (T1, seedling 2); 10P3 TBSJ1 T10 (T1, seedling 3); UP untransformed potato; TP transgenic potato; TPT Transgenic potato tuber; CFA culture filtrate antigen
ELISA
The ELISA was done to quantify the levels of the recombinant GroES (rGroES) protein expression in tobacco and potato. Of the three tobacco T0 plant proteins tested, TBSJ1 T8 gave the highest expression of the recombinant protein at 0.28 % of TSP, with T10 recording only 0.04 % and T26 showing a negative result (Fig. 9a). The expression levels of rGroES varied among the progeny tested of the two transgenic lines T8 and T10. One of the T8 progeny (8P1) recorded the highest expression of 1.2 % of TSP. The levels of rGroES expression in the progeny of both lines were comparatively higher than the parent. The progeny 10P3 of T10 parent showed up to 13-fold increase from that of its parent. The yields of the rGroES protein in 8P2 and 8P3 in (Fig. 9 B) were high. Among the T8 progeny 8P2 recorded 12 µg/mg TSP and the highest among the T10 progeny was 5 µg/mg TSP. In terms of yield per gram leaf tissue, 8P2 recorded about 72 µg of rGroES protein with 8P3 giving about 69 µg (Fig. 9c). Of the protein samples tested from control and transgenic potato, only the potato transgenic tuber recorded a comparatively low yield of 0.33 µg of the rGroES protein for a milligram of TSP.
Discussion
Over the last decade, transgenic plants have proved to be a valuable, economic, and contamination free production platform of heterologous proteins (Ceasar and Ignacimuthu 2009). Plants, being eukaryotes, are potential bioreactors for production of biologically active therapeutic proteins. However, the choice of the plant system would depend upon the application of the recombinant protein.
We selected the highly immunodominant M. tuberculosis chaperonin 10 (cpn10, GroES) as a candidate TB antigen for expression in tobacco and potato. The chaperonin 10 from M. tuberculosis and from M. leprae produced strong immune responses in tuberculosis or leprosy patients (Mehra et al. 1992). With several TB antigens having been predicted as possible candidates (Mattow et al. 2003; Bellinzoni and Riccardi 2003), a few of those antigens namely ESAT-6 and the Ag85B have been expressed either transiently or stably in plants (Rigano et al. 2004; Zelada et al. 2006; Dorokhov et al. 2007). In prokaryotes Piubelli et al. (2013) showed the expression of TB10.4, Ag85B and TB10.4-Ag85B antigens in E. coli, Lu et al. (2007) showed the expression of recombinant Ag85B antigen in E. coli strains JM109DE3 and Origami B, Dhar et al. (2004) showed the expression of Ag85A and Ag85C antigens in recombinant BCG strains and Steyn et al. (2003) showed the expression of Rv1368 and Rv1690 antigens in yeast.
Young and Garbe (1991), identified the M. tuberculosis cpn10 as a major T cell antigen during infection. It is one of the most abundant proteins in the culture filtrate (Abou-Zeid et al. 1988). On infection, the pathogen is under stress producing a slew of heat shock proteins (Hsps); Chaperonin 10 (Hsp 10) being a Hsp, will have the Hsp-derived peptides well represented in the host milieu eliciting a strong immune response in the host (Qamra et al. 2005). The 10 kDa chaperonin, present in the phagosome is an important protein for the survival of the Mycobacterium in the macrophage, its persistence and virulence (Bellinzoni and Riccardi 2003). In a study comparing the proteomes of the virulent M. tuberculosis H37Rv strain with the attenuated M. bovis BCG Copenhagen, Mattow et al. (2003) have predicted the 10 kDa Chaperonin as one of the 27 candidate antigens for novel vaccines and diagnostics in TB research. These included five proteins encoded by open reading frames and 22 novel differential proteins, such as acetyl-CoA C-acetyltransferase (Rv0243) and two putative Esat6-like proteins (Rv1198, Rv1793). In the resent study the GroES mycobacterial gene sequenced without codon optimisation, except for the N-terminal GTG that was altered to ATG (eukaryote translation start site, TSS), was used for expression in tobacco and potato.
The MALDI-MS data and an in silico MASCOT search confirmed the identity of the plant derived recombinant protein as the 10 kDa Chaperonin from M. tuberculosis and also gave the PMF of the analysed protein. The Mowse scores were significant indicating the probability that the matches were high. The MS/MS data confirmed the microsequence of the tryptic peptide precursor ions. In our study, the MALDI-TOF/TOF results were the first and most substantive evidence of the recombinant M. tuberculosis GroES gene expression in tobacco. The immuno-dot blot assay was a preliminary qualitative test to detect the levels of protein expression in T1 progeny and to make a comparison with its T0 parent line. ELISA estimated the rGroES TB antigen concentration in T0 lines and T1. The T1 progeny showed several fold higher concentration of the TB antigen. The screening of more of the T1 progeny could result in the identification of plants with higher yields.
We have successfully engineered the GroES mycobacterial gene sequence without codon optimisation, except for the N-terminal GTG that was altered to ATG (eukaryote translation start site, TSS), for expression in a eukaryotic plant system. The comparatively high recombinant protein expression levels in our study using tobacco as an expression system may be attributed to two factors. The use of the (1) TMV 5′ leader sequence (Ω), cloned along with (2) Kozak sequence in the right context (both are known to enhance translation), resulting in an increase of protein production by several fold. Gallie (2002), demonstrated in tobacco protoplasts that the 5′-leader of the tobacco mosaic virus (Ω) enhances translation by recruiting the eIF4F translation factor. Tobacco, a non-food crop, is a strong candidate crop for the commercial production of recombinant proteins (Stoger et al. 2002) and has been used in Cuba for the commercial production of the recombinant antibody against hepatitis B (Ramirez et al. 2003; Valdés et al. 2003; Pujol et al. 2005). However, the alkaloid content in tobacco being a deterrent for consideration as a crop for the expression of a putative edible vaccine, a popular commercial Indian variety of potato cv. Kufri bahar, using shoot tips was also selected for Agrobacterium-mediated transformation in our study. This confirms our previous report that the shoot tips explant was a good choice for plant regeneration and transformation (Ceasar and Ignacimuthu 2010; Ramakrishnan et al. 2013).
In comparison to tobacco the rGroES gene expression in potato was low. The recombinant GroES protein was detected only in the in vitro microtubers. The ELISA absorbance readings for the transgenic potato plant and that of the untransformed potato plant TSP were almost the same and hence treated as nil expression. However, the expression levels of the foreign protein in the microtubers in our study were nearly the same as the expression levels of other candidate vaccines in potato (Arakawa et al. 2001; Kim and Langridge 2003; Shulga et al. 2004). Shulga et al. (2004) reported that transgenic potato plants expressing the gene of hepatitis B surface antigen (HBsAg) under the control of the double promoter of 35S cauliflower mosaic virus (CaMV 35SS) yielded HBsAg at a concentration of 0.005–0.035 % of TSP. They reported a marginal increase of expression in in vitro raised microtubers. Castañón et al. (2002) used five different promoter constructs to compare their efficacy in the expression of the VP 60 gene from the rabbit haemorrhagic disease virus in potato plants. The rabbits fed with the plant derived VP 60 protein showed protective immunity on challenge. Some of the subunit vaccines produced in potatoes have undergone clinical trials in humans and had given gratifying results. High levels of antibody production have been achieved in transgenic potato tubers (Artsaenko et al. 1998). Potatoes can be used for the production of plant made pharmaceuticals as the product remains stable and this may be attributed to the specialised molecular environment in the tuber.
We have demonstrated for the first time by stable transformation, the expression of the M. tuberculosis immunodominant 10 kDa chaperonin in tobacco and potato. The levels of expression in both tobacco and potato using non-viral binary vectors have been comparable with other studies. A further codon optimisation for protein expression in a eukaryote and the use of viral based vectors may further enhance the expression of the GroES TB antigen in tobacco and potato. Plants yielding the foreign protein at a rate greater than 1 % of the TSP are better poised for recombinant protein purification and commercial exploitation and plant-derived biopharmaceuticals are cheap to produce and store, easy to scale up for mass production, and safer than those derived from animals (Daniell et al. 2001; Teli and Timko 2004). Numerous plant species have been used for antigen expression, including tobacco, potato, tomato, banana, corn, lupine, green algae and lettuce (Walmsley and Arntzen, 2000; Carter and Langridge, 2002; Sala et al., 2003; Valdes et al. 2012; Rasala and Mayfield 2014) and the list of protective antigens from microbial and viral pathogens that have been expressed in plants has become increasingly larger. The development of edible oral vaccines is certainly among the most exciting current research areas (Teli and Timko 2004).
In view of using this plant-made recombinant GroES antigen as a subunit vaccine we performed an in silico search to identify its antigenic determinants. Employing one of the MHCBN database tools we were able to identify and map the T-cell epitope. This database provided information that confirmed the source. It identified the MHC class II allele HLA-DRB1*1405 that had the binding site for the identified T-cell epitope. They also provided information on the T-cell binding and proliferation assays that were positive.
Change history
18 September 2017
The lower panel in Fig. 2 of the original publication does not show the gels of the correct experiment. The correct lower panel is printed below, incl. caption. The results of the correct experiment do not change the interpretation of the findings of the work and the conclusions remain valid.
Abbreviations
- TB:
-
Tuberculosis
- TSP:
-
Total soluble protein
- BCG:
-
Bacille Calmette-Guérin
- CaMV:
-
Cauliflower mosaic virus
- Cpn 10:
-
Chaperonin 10
- MALDI-TOF:
-
Matrix assisted laser desorption ionisation time-of-flight
- MS:
-
Murashige and Skoog
- BA:
-
Benzyladenine
- NAA:
-
Naphthalene acetic acid
- GA3 :
-
Gibberellic acid
- Hyg:
-
Hygromycin B
- ACN:
-
Acetonitrile
- TFA:
-
Trifluoroacetic acid
- PVDF:
-
Polyvinylidene fluoride
- NTM:
-
Nicotiana tabacum medium
- NTR:
-
Nicotiana tabacum regeneration
- STS:
-
Solanum tuberosum stem
- STT:
-
Solanum tuberosum transformation
- STI:
-
Solanum tuberosum internodes
- STN:
-
Solanum tuberosum node
References
Abou-Zeid C, Smith I, Grange JM, Ratliff TL, Steele J, Rook GA (1988) The secreted antigens of Mycobacterium tuberculosis and their relationship to those recognized by the available antibodies. J Gen Microbiol 134:531–538
Ahmed N, Caviedes L, Alam M, Rao KR, Sangal V, Sheen P, Gilman RH, Hasnain SE (2003) Distinctiveness of Mycobacterium tuberculosis genotypes from human immunodeficiency virus type 1-seropositive and -seronegative patients in Lima, Peru. J Clin Microbiol 41:1712–1716
Arakawa T, Yu J, Langridge WHR (2001) Synthesis of a cholera toxin B subunit-rotavirus NSP4 fusion protein in potato. Plant Cell Rep 20:343–348
Artsaenko O, Kettig B, Fiedler U, Conrad U, During K (1998) Potato tubers as a biofactory for recombinant antibodies. Mol Breed 4:313–319
Arvindhan (2005) Construction of recombinant BCG Based HIV-1 epitope delivery system, in Department of Immunology, Tuberculosis Research Centre, Chennai, University of Madras
Baird PN, Hall LM, Coates AR (1989) Cloning and sequence analysis of the 10 kDa antigen gene of Mycobacterium tuberculosis. J Gen Microbiol 135:931–939
Barnes PF, Mehra V, Rivoire B, Fong SJ, Brennan PJ, Voegtline MS, Minden P, Houghten RA, Bloom BR, Modlin RL (1992) Immunoreactivity of a 10-kDa antigen of Mycobacterium tuberculosis. J Immunol 148:1835–1840
Bellinzoni M, Riccardi G (2003) Techniques and applications: the heterologous expression of Mycobacterium tuberculosis genes is an uphill road. Trends Microbiol 11:351–358
Berendzen K, Searle I, Ravenscroft D, Koncz C, Batschauer A, Coupland G, Somssich IE, Ulker B (2005) A rapid and versatile combined DNA/RNA extraction protocol and its application to the analysis of a novel DNA marker set polymorphic between Arabidopsis thaliana ecotypes Col-0 and Landsberg erecta. Plant Methods 1:4
Bloom BR, Murray CJ (1992) Tuberculosis: commentary on a re-emergent killer. Science 257:1055–1064
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carter JE, Langridge WHR (2002) Plant-based vaccines for protection against infectious and autoimmune diseases. Crit Rev Plant Sci 21:93–109
Castañón S, Martin-Alonso JM, Marin MS, Boga JA, Alonso P, Parra F, Ordas R (2002) The effect of the promoter on expression of VP60 gene from rabbit hemorrhagic disease virus in potato plants. Plant Sci 162:87–95
Ceasar SA, Ignacimuthu S (2009) Genetic engineering of millets: current status and future prospects. Biotechnol Lett 31:779–788
Ceasar SA, Ignacimuthu S (2010) Effects of cytokinins, carbohydrates and amino acids on induction and maturation of somatic embryos in kodo millet (Paspalum scorbiculatum Linn.). Plant Cell Tiss Organ Cult 102:153–162
Chakhaiyar P, Hasnain SE (2004) Defining the mandate of tuberculosis research in a postgenomic era. Med Princ Pract 13:177–184
Cohn DL, Bustreo F, Raviglione MC (1997) Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD Global Surveillance Project. International Union against Tuberculosis and Lung Disease. Clin Infect Dis 24(Suppl 1):S121-S130
Daniell H, Streatfield SJ, Wycoff K (2001) Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci 6:219–226
Dhar N, Rao V, Tyagi AK (2004) Immunogenicity of recombinant BCG vaccine strains overexpressing components of the antigen 85 complex of Mycobacterium tuberculosis. Med Microbiol Immunol 193:19–25
Dorokhov YL, Sheveleva AA, Frolova OY, Komarova TV, Zvereva AS, Ivanov PA, Atabekov JG (2007) Superexpression of tuberculosis antigens in plant leaves. Tuberculosis (Edinb) 87:218–224
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissues. Focus 12(1):13–15
Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC (1999) Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677–686
Dye C, Espinal MA, Watt CJ, Mbiaga C, Williams BG (2002) Worldwide incidence of multidrug-resistant tuberculosis. J Infect Dis 185:1197–1202
Fine PE (1989) The BCG story: lessons from the past and implications for the future. Rev Infect Dis 11(Suppl 2):S353–S359
Gallie DR (2002) The 5′-leader of tobacco mosaic virus promotes translation through enhanced recruitment of eIF4F. Nucleic Acids Res 30:3401–3411
Kim TG, Langridge WH (2003) Assembly of cholera toxin B subunit full-length rotavirus NSP4 fusion protein oligomers in transgenic potato. Plant Cell Rep 21:884–890
Lu D, Contreras LG, Xu D, Kurtz SL, Liu J, Braunstein M, Mcmurray DN, Hickey AJ (2007) Poly (lactide-co-glycolide) microspheres in respirable sizes enhance an in vitro T Cell response to recombinant Mycobacterium tuberculosis antigen 85B. Pharm Res 24:10
Mattow J, Schaible UE, Schmidt F, Hagens K, Siejak F, Brestrich G, Haeselbarth G, Muller EC, Jungblut PR, Kaufmann SH (2003) Comparative proteome analysis of culture supernatant proteins from virulent Mycobacterium tuberculosis H37Rv and attenuated M. bovis BCG Copenhagen. Electrophoresis 24:3405–3420
Mehra V, Bloom BR, Bajardi AC (1992) A major T cell antigen of Mycobacterium leprae is a 10 kDa heat shock cognate protein. J Exp Med 175:275–284
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Orme IM, McMurray DN, Belisle JT (2001) Tuberculosis vaccine development: recent progress. Trends Microbiol 9:115–118
Piubelli L, Campa M, Temporini C, Binda E, Mangione F, Amicosante M, Terreni M, Marinelli F, Pollegioni L (2013) Optimizing Escherichia coli as a protein expression platform to produce Mycobacterium tuberculosis immunogenic proteins. Microb Cell Factories 12:115
Pujol M, Ramirez NI, Ayala M, Gavilondo JV, Valdes R, Rodriguez M, Brito J, Padilla S, Gomez L, Reyes B, Peral R, Perez M, Marcelo JL, Mila L, Sanchez RF, Paez R, Cremata JA, Enriquez G, Mendoza O, Ortega M, Borroto C (2005) An integral approach towards a practical application for a plant-made monoclonal antibody in vaccine purification. Vaccine 23:1833–1837
Qamra R, Mande SC, Coates AR, Henderson B (2005) The unusual chaperonins of Mycobacterium tuberculosis. Tuberculosis (Edinb) 85:385–394
Ramakrishnan M, Ceasar SA, Duraipandiyan V, Melvin AD, Ignacimuthu S (2013) Efficacious somatic embryogenesis and fertile plant recovery from shoot apex explants of onion (Allium cepa. L.). In Vitro Cell Dev Biol Plant 49:285–293
Ramirez N, Rodriguez M, Ayala M, Cremata J, Perez M, Martinez A, Linares M, Hevia Y, Paez R, Valdes R, Gavilondo JV, Selman-Housein G (2003) Expression and characterization of an anti-(hepatitis B surface antigen) glycosylated mouse antibody in transgenic tobacco (Nicotiana tabacum) plants and its use in the immunopurification of its target antigen. Biotechnol Appl Biochem 38:223–230
Rasala BA, Mayfield SP (2014) Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth Res. doi:10.1007/s11120-014-999-7
Rigano MM, Alvarez ML, Pinkhasov J, Jin Y, Sala F, Arntzen CJ, Walmsley AM (2004) Production of a fusion protein consisting of the enterotoxigenic Escherichia coli heat-labile toxin B subunit and a tuberculosis antigen in Arabidopsis thaliana. Plant Cell Rep 22:502–508
Sala F, Manuela Rigano M, Barbante A, Basso B, Walmsley AM, Castiglione S (2003) Vaccine antigen production in transgenic plants: strategies, gene constructs and perspectives. Vaccine 21:803–808
Sambrook J, Fritschi EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Shulga NY, Rukavtsova EB, Krymsky MA, Borisova VN, Melnikov VA, Bykov VA, Buryanov YI (2004) Expression and characterization of hepatitis B surface antigen in transgenic potato plants. Biochemistry (Mosc) 69:1158–1164
Siddiqi N, Shamim M, Hussain S, Choudhary RK, Ahmed N, Prachee, Banerjee S, Savithri GR, Alam M, Pathak N, Amin A, Hanief M, Katoch VM, Sharma SK, Hasnain SE (2002) Molecular characterization of multidrug-resistant isolates of Mycobacterium tuberculosis from patients in North India. Antimicrob Agents Chemother 46:443–450
Steyn AJC, Joseph J, Bloom BR (2003) Interaction of the sensor module of Mycobacterium tuberculosis H37Rv KdpD with members of the Lpr family. Mol Microbiol 47(4):1075–1089
Stoger E, Sack M, Fischer R, Christou P (2002) Plantibodies: applications, advantages and bottlenecks. Curr Opin Biotechnol 13:161–166
Teli PN, Timko MP (2004) Recent developments in the use of transgenic plants for the production of human therapeutics and biopharmaceuticals. Plant Cell, Tissue Organ Cult 79:125–145
Valdes R et al (2012) Production of a monoclonal antibody by ascites, hollow fiber system, and transgenic plants for vaccine production using CB.Hep-1 mAb as a study case. Biotechnol Bioprocess Eng 17(1):145–159
Valdés R, Gómez L, Padilla S, Brito J, Reyes B, Alvarez T, Mendoza O, Herrera O, Ferro W, Pujol M, Leal V, Linares M, Hevia Y, García C, Mila L, García O, Sánchez R, Acosta A, Geada D, Paez R, Vega JL, Borroto C (2003) Large-scale purification of an antibody directed against hepatitis B surface antigen from transgenic tobacco plants. Biochem Biophys Res Commun 308:94–100
Walmsley AM, Arntzen CJ (2000) Plants for delivery of edible vaccines. Curr Opin Biotechnol 11:126–129
Young DB, Garbe TR (1991) Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect Immun 59:3086–3093
Zelada AM, Calamante G, de la Paz Santangelo M, Bigi F, Verna F, Mentaberry A, Cataldi A (2006) Expression of tuberculosis antigen ESAT-6 in Nicotiana tabacum using a potato virus X-based vector. Tuberculosis (Edinb) 86:263–267
Acknowledgments
We gratefully acknowledge the supply of the GroES TB antigen standards and primary monoclonal antibodies by Prof. John Belisle, TB Vaccine Testing and Research Materials Contract at the Colorado State University. We also acknowledge with thanks the assistance by Prof. Dipanker Chatterjee and S. Prakash in performing the MALDI-TOF TOF analysis at Molecular Biophysics Unit, Indian Institute of Science, Bangalore, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1007/s11240-017-1303-7.
Rights and permissions
About this article
Cite this article
Jose, S., Ignacimuthu, S., Ramakrishnan, M. et al. Expression of GroES TB antigen in tobacco and potato. Plant Cell Tiss Organ Cult 119, 157–169 (2014). https://doi.org/10.1007/s11240-014-0522-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0522-4