Abstract
Rivaroxaban is a direct, oral factor Xa inhibitor that is used for the prevention and treatment of various thromboembolic disorders. Several preclinical and clinical studies have utilized specific molecules as biomarkers to investigate the potential role of rivaroxaban beyond its anticoagulant activity and across a range of biological processes. The aim of this review is to summarize the existing evidence regarding the use of blood-based biomarkers to characterize the effects of rivaroxaban on coagulation and other pathways, including platelet activation, inflammation and endothelial effects. After a literature search using PubMed, almost 100 preclinical and clinical studies were identified that investigated the effects of rivaroxaban using molecular biomarkers. In agreement with the preclinical data, clinical studies reported a trend for reduction in the blood concentrations of D-dimers, thrombin–antithrombin complex and prothrombin fragment 1 + 2 following treatment with rivaroxaban in both healthy individuals and those with various chronic conditions. Preclinical and also some clinical studies have also reported a potential impact of rivaroxaban on the concentrations of platelet activation biomarkers (von Willebrand factor, P-selectin and thrombomodulin), endothelial activation biomarkers (matrix metalloproteinase-9, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1) and inflammation biomarkers (interleukin-6, tumor necrosis factor-α and monocyte chemoattractant protein-1). Based on the results of biomarker studies, molecular biomarkers can be used in addition to traditional coagulation assays to increase the understanding of the anticoagulation effects of rivaroxaban. Moreover, there is preliminary evidence to suggest that rivaroxaban may have an impact on the biological pathways of platelet activation, endothelial activation and inflammation; however, owing to paucity of clinical data to investigate the trends reported in preclinical studies, further investigation is required to clarify these observations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Rivaroxaban, a direct, oral factor Xa inhibitor used for the prevention and treatment of various thromboembolic disorders potentially plays a role beyond its anticoagulant activity and across a range of biological processes.
-
A literature search with the main focus on Medline via PubMed was done to summarize the existing evidence regarding the use of blood-based biomarkers to characterize the effects of rivaroxaban on coagulation and other pathways, including platelet activation, inflammation and endothelial effects in clinical and preclinical studies.
-
In preclinical and clinical studies not only a trend for reduction in the blood concentrations of some relevant coagulation biomarkers (D-dimers, thrombin–antithrombin complex and prothrombin fragment 1+2) following treatment with rivaroxaban was reported, but also for a potential impact of rivaroxaban on the concentrations of platelet activation biomarkers (von Willebrand factor, P-selectin and thrombomodulin), endothelial activation biomarkers (matrix metalloproteinase-9, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1) and inflammation biomarkers (interleukin-6, tumor necrosis factor-α and monocyte chemoattractant protein-1).
-
Clinical impact of those findings is work in progress.
Background
The coagulation cascade consists of a sequence of consecutive protease activations steps ultimately leading to the generation of fibrin and contributing to platelet activation, the two main processes in blood clotting. A well-functioning coagulation cascade is central to maintaining hemostasis [1]. Conditions which can lead to prothrombotic states, including diseases like atrial fibrillation (AF) and acute coronary syndrome (ACS) or procedures resulting in immobility, such as hip or knee surgery [2,3,4] may cause hypercoagulability increasing the risk of development of life-threatening thrombosis [1, 5].
Rivaroxaban is a direct, oral anticoagulant that is used for the prevention and treatment of various thromboembolic disorders. The mode of action involves the reversible inhibition of activated factor Xa (FXa), a key component of the blood coagulation pathway [6, 7]. Licensed indications for rivaroxaban include: Treatment of pulmonary embolism (PE) or deep vein thrombosis (DVT); prevention of recurrence of PE or DVT; prevention of systemic embolism or stroke in patients with nonvalvular AF; prophylaxis of VTE in patients undergoing hip or knee replacement surgery and prevention of atherothrombotic events after ACS and in CAD/symptomatic PAD [2, 8,9,10].
Coagulation factors have also been implicated in other biological processes, such as tissue repair, platelet activation and inflammation [11, 12]. As such, it has been hypothesized that rivaroxaban could have additional impacts on a range of those biological processes. One approach that can be used to investigate the potential effects of rivaroxaban on other biological pathways is through the measurement of biomarkers, molecules known to provide insights into the status of these processes. Studies of biomarkers can provide information regarding ongoing biological changes and can improve understanding of the mode of action of drugs. Disease-related and drug-related pharmacodynamic biomarkers have been used to help understand and predict patients’ characteristics and risk, optimize patients’ selection, drug dosing and improve decision-making throughout the drug development process [13]. Furthermore, the capabilities of assays in the preclinical setting have allowed for the exploratory investigation of potential biomarkers to understand a variety of processes which could potentially be translated into the clinic.
The aim of this literature review is to summarize the evidence for the impact of rivaroxaban on various pathways; specifically, we describe studies exploring how molecular biomarkers may be used to further characterize the effects of rivaroxaban on coagulation and on other biological processes.
Methods
To inform the discussions in this review, searches were conducted in Medline mainly via PubMed to identify literature reporting preclinical and clinical studies of rivaroxaban and biomarkers of coagulation, platelet activation, inflammation, endothelial changes and other biological processes. The following search strings were used.
-
1.
(rivaroxaban) AND (biomarker) [All Fields] (January 2022)
-
2.
(rivaroxaban[Title/Abstract]) AND ((oxidation[Title] OR oxidant[Title] OR platelet[Title] OR endothelial[Title]) OR (inflammation[Title] OR inflammatory[Title] OR cytokine[Title] OR leukocyte[Title]) OR (coagulant[Title] OR coagulation[Title] OR d dimer[Title] OR prothrombin[Title] OR viper venom[Title])) (January 2022)
The references retrieved from these searches were screened and a total of 97 studies were identified. The search results were confirmed by similar search terms in other databases including Google Scholar. In addition to these searches, reference lists from published literature reviews were cross-checked to identify studies that discussed disease biomarkers for various indications of rivaroxaban (January 2022).
The coagulation cascade
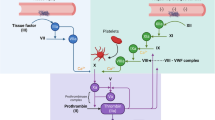
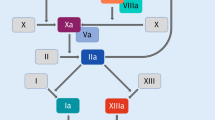
The coagulation cascade is a complex sequence of events. Blood clotting is initiated when either subendothelial tissue factor (TF) gets into contact with blood after vessel wall injury, binds activated factor VIIa (FVIIa) and activates factor IX and factor X (FX) [7] or in the cause of contact activation, i.e. FXII is activated on negatively charged surfaces, leading to factor XI- and FIX activation. As a result of such signaling, FX is activated to FXa and catalyzes thrombin generation by acting as part of the prothrombinase complex; thrombin is subsequently responsible for the conversion of fibrinogen to fibrin, a central process in blood clot formation [14]. Rivaroxaban has been shown to inhibit FXa, irrespective of whether it is free or bound in the prothrombinase complex, resulting in reduced thrombin generation, thereby prolonging blood clotting times [14,15,16].
Global/functional assays of rivaroxaban activity
Various assays can be used to assess the functionality of the coagulation cascade. While most of these assays use the development of a fibrin clot as common endpoint, it is the trigger and thereby the starting point, which varies. Some assays like the prothrombin time (used to either measure the extrinsically triggered coagulation or monitor the impact of Vitamin K antagonists), or activated partial thromboplastin time (used to either measure the intrinsic pathway or the impact of heparin therapy) are among the most frequently performed tests in the clinical laboratory and are available even through handheld self-testing devices. Other methods are rarely used—only to focus on specific questions. While most of those assays are usually performed from anticoagulated (mostly citrated) plasma, ROTEM (rotational thromboelastometry) and TEG (thromboelastography) are whole blood tests, which are most often used in critical care setting. The thrombin generation assay, usually only available in specialized labs, allows to determine the formation of thrombin as key enzyme of the coagulation over time, thereby providing better insights into kinetic aspects of coagulation.
Figure 1 provides an overview of these types of methods and the stages of the coagulation pathways that they measure. From the earliest experiments with rivaroxaban on it has been known that the compound prolongs the clotting times dose-dependently. Table 1 lists examples of studies that have reported the anticoagulation effects of rivaroxaban, specifically through the use of functional coagulation assays [10, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
Molecular biomarkers of rivaroxaban activity
In addition to the use of global/functional assays to assess the coagulation status, the impact of rivaroxaban has been investigated by measuring concentrations of specific biomarkers, see Fig. 2. Molecular biomarkers may provide supplementary information regarding the effects of rivaroxaban because they allow for a snapshot of the concentrations of coagulation products. Examples of the investigated molecular biomarkers are depicted in Fig. 2 and include different types:
-
Direct markers: Biomarkers, that are directly affected by the mode-of-action of rivaroxaban, i.e. as a proximate result of FXa inhibition and subsequent reduction of thrombin formation.
-
Indirect markers: Biomarkers that indicate biological processes downstream of FXa and thrombin, either within the coagulation cascade (e.g. D-Dimer) or outside (e.g. inflammatory markers).
Direct markers are directly affected by the mode-of-action of rivaroxaban, i.e. as a proximate result of FXa inhibition and subsequent reduction of thrombin formation. Indirect markers indicate biological processes downstream of FXa and thrombin, either within the coagulation cascade (e.g. D-Dimer) or outside (e.g. inflammatory markers).
F1 + 2, prothrombin fragment 1 + 2; FXa, activated factor X; ICAM-1, intercellular adhesion molecule-1; IL-1β, interleukin 1β; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; MMP-9, matrix metalloproteinase-9; NOS2; nitric oxide synthase isotope 2; PAR, protease-activated receptor; TAT, thrombin–antithrombin complex; β-ThG, thromboglobulin; TM, thrombomodulin; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1; vWF, von Willebrand factor.
Direct biomarkers
The biomarkers Russel’s viper venum (RVV) FX test, prothrombin fragment 1 + 2 (F1 + 2) and thrombin–antithrombin (TAT) complex are directly derived from substrates of the activated coagulation pathway by FXa. Studies have investigated the effects of rivaroxaban on the concentrations of these molecular biomarkers.
Russell’s viper venom (RVV) FX test.
Russell's Viper Venom is an enzyme extracted from snake venom (Daboia russelii) and activates Factor X directly. In the presence of Factor V, Prothrombin, Calcium and Phospholipid a fibrin clot is formed. Within the assay, Factor Xa specifically cleaves a substrate which can be optically measured. This reaction can be used to directly assess the effect of Rivaroxaban on FXa activity in plasma. Hence, it was shown clinically that Russell viper venom reagents correlate with rivaroxaban concentration and that they can reflect the inhibition of FXa in pre-clinical models [16, 47].
Prothrombin fragment 1 + 2 (F1 + 2).
F1 + 2 is a peptide cleaved from the amino-terminal end of prothrombin by FXa during activation to thrombin. As such, the concentration of F1 + 2 in the plasma reflects in vivo thrombin generation [48].
There is some clinical evidence of F1 + 2 as a useful molecular biomarker to assess the effect of rivaroxaban. Several clinical studies have reported reductions from baseline in the plasma concentration of F1 + 2 in rivaroxaban-treated patients with heart failure (HF) [19], AF [27, 49] or acute cardioembolic stroke [22], as well as in healthy individuals [50, 51] and in patients after hip or knee replacement surgery [21]. For example, in a study conducted by Gheorghiade et al. [19], in patients with HF who were treated with rivaroxaban, the mean concentration of F1 + 2 decreased by 2.7 ng/mL over 7 days compared with an increase of 11.6 ng/mL in patients who received placebo (p = 0.0009 for the difference between groups) [19]. Similarly, a study in patients with AF reported lower mean concentrations of F1 + 2 in circulating plasma in patients treated with rivaroxaban (103.5 pmol/L) than in patients not treated with anticoagulants (162.5 pmol/L) [52]. However, not all studies have reported this trend: the study of Miyazawa et al. [53] in patients with nonvalvular AF and left atrial/left atrial appendage (LA/LAA) thrombus, who received rivaroxaban 20 mg once daily, found no significant baseline-adjusted differences in the mean concentrations of F1 + 2 [53].
Studies have also compared the respective effects of warfarin and rivaroxaban treatment on plasma levels of F1 + 2 in patients with AF. These investigations reported lower plasma levels of F1 + 2 in warfarin-treated patients than in rivaroxaban-treated patients. One hypothesis for this finding is that the pharmacokinetic profiles of the two compounds might lead to differences in the interaction with the kinetics of F1 + 2, e.g. mean half-life of Rivaroxaban is 5–13 h and of Warfarin 40 h. [24, 36, 54]. Similarly, a study in patients with cardioembolic stroke receiving rivaroxaban or warfarin for secondary stroke prevention did not find any rivaroxaban-treated patients with levels of F1 + 2 below the normal range, irrespective of dosage. However, many patients receiving warfarin had levels below the normal range. As such, the authors proposed that normal thrombin generation may be preserved even when rivaroxaban treatment is at its peak level; this could partly explain the observed, favorable outcomes in rivaroxaban-treated patients with intracranial hemorrhage [22].
Thrombin–antithrombin III complex.
Levels of thrombin are regulated by a variety of physiological inhibitors; the main inhibitor of thrombin is antithrombin III (ATIII) [55, 56]. Antithrombin and thrombin form equimolar thrombin-antithrombin III complexes (TAT) which lead to the inactivation of thrombin in blood. The concentrations of TAT in the blood have been shown to reflect the formation of thrombin [56, 57].Preclinical evidence in animal models generally supports the use of TAT complex concentrations as a biomarker of the anticoagulation effects of rivaroxaban. In some rat and mouse models of hypercoagulation, plasma concentrations of TAT were lower in rivaroxaban-treated animals than in controls [40, 42, 58]. For example, in a rat model of brain ischemia/reperfusion injury reported by Dittmeier et al. [58], mean concentrations of TAT in the brain were significantly lower 1 day after stroke in rats pretreated with rivaroxaban than in vehicle-treated controls (3057.0 pg/mL vs 5048.0 pg/mL; p < 0.05) [58]. Conversely, in a study of apolipoprotein E-deficient mice, Hara et al. [59] reported no difference in plasma concentrations of TAT between rivaroxaban-treated mice and controls [59]. The impact of rivaroxaban on the TAT concentrations in these different animal experiments may depend on the dose of rivaroxaban, the animal species used in the study and importantly the different pathologies involved. For instance, thrombosis studies with high TAT levels in the control group share higher chances to yield significant TAT level reductions with rivaroxaban, whereas experiments yielding low TAT levels in the control group, like atherosclerotic experiments without thrombotic event, may result in low TAT reduction levels with rivaroxaban.
As anticipated by the preclinical evidence, clinical studies have also reported a trend for reduction in TAT complex levels following treatment with rivaroxaban. Reductions in the plasma concentrations of TAT complex from baseline after rivaroxaban treatment have been observed in patients with nonvalvular AF [53, 54] in healthy individuals [50] and in patients after hip or knee replacement surgery [21]. In the study of healthy individuals conducted by Weisshaar et al. [50], single doses of rivaroxaban (combined with ticagrelor and acetylsalicylic acid) significantly reduced concentrations of TAT complex in shed blood at 3 h after treatment (median, 127 µg/L at 3 h vs 630 µg/L at baseline; p < 0.001) [50]. Similarly, in a study of rivaroxaban-treated patients undergoing percutaneous coronary intervention (PCI), concentrations of TAT complex were suppressed after the PCI [38].
Protease-activated receptors-1, -2 and -4.
Initiation of the coagulation cascade results in the activation of platelets and upregulation of adhesion molecules and pro-inflammatory pathways in blood, which in turn accelerates the coagulation processes. Key to this signaling is the cleavage of peptides from protease-activated receptors by FXa and thrombin liberating the tethered ligands, which activate the respective receptor. Thrombin activates by this means protease-activated receptors-1 and -4 (PAR-1 and PAR-4) and FXa PAR-2 and (weakly) PAR-1 (Fig. 2) [60, 61]. In addition, PAR-2 seems to be activatable by the TF-FVIIa-FXa complex. [62, 63]. Because the receptor activation leads to subsequent internalization, this process cannot be measured easily on the cell surface but might be monitored at later stages of the signaling cascade. The activation of PAR-1 and -4 on platelets leads to their activation, while activation of PAR-1 and -2 receptors on endothelial cells and various other cell types results in proinflammatory signaling through several pathways, which trigger among others the generation of pro-inflammatory molecules.
Preclinical studies have reported some associations between rivaroxaban treatment and a reduction in the concentrations of PARs. For example, in a mouse model of myocardial reperfusion injury, compared with controls, mice treated with rivaroxaban had significantly reduced levels of mRNA for PAR-2 in the left ventricle. Chung et al. showed in an atrial fibrosis model with isoproterenol-treated rats that rivaroxaban decreases collagen production and migratory capability of atrial fibroblasts by increasing NO production and decreasing Ca2 + entry through inhibition of PAR signaling [64]. In vitro studies showed that DOACs, including rivaroxaban, seem to limit the alteration of the monolayer of endothelial cells of the blood brain barrier mediated by the thrombin/PAR-1 pathway [65]. Furthermore it was suggested, that rivaroxaban-mediated inhibition of PAR-1 has a positive impact on atherothrombotic events [66].
Indirect biomarkers
Indirect biomarkers can provide insights into biological pathways that are also affected by various players downstream in the coagulation cascade, and thereby provide a window into other processes that may be affected by rivaroxaban. Examples of such molecular markers are depicted in Fig. 2. The following sections summarize the published evidence for the impact of rivaroxaban on molecular biomarkers of fibrin formation and coagulation, platelet activation, inflammation and endothelial changes.
D-dimers
D-dimers derive from the cleavage of cross-linked, insoluble fibrin molecules during endovascular thrombosis, one of the last stages in the coagulation cascade. Serum/plasma D-dimer levels have been shown to correlate with extent of thrombolytic activity in the body and the amount of thrombotic deposits [67, 68].
Many clinical studies have demonstrated a reduction in the concentrations of D-dimers in the blood following treatment with rivaroxaban. Reductions in plasma concentrations of D-dimer from baseline after rivaroxaban treatment have been reported in patients with AF [26, 27, 53], ACS [69] or acute cardioembolic stroke [22], as well as in healthy individuals [70]. For example, in a sub-study of patients with ACS from the ATLAS ACS-TIMI 46 trial, reductions from baseline in median D-dimer levels 180 days after treatment initiation were significantly greater (p < 0.001) in the rivaroxaban-treated group (− 0.14 µg/mL) than with placebo (− 0.06 µg/mL) [69]. Similarly, in the X-TRA biomarker sub-study of patients with LA/LAA thrombus and AF, patients treated with rivaroxaban had a reduction in plasma concentrations of D-dimer from baseline to the end of treatment (− 41.5%; p < 0.001) [53]. In the X-VeRT substudy which evaluated the effects of treatment with rivaroxaban or VKA on levels of different biomarkers of coagulation and inflammation in nonvalvular AF patients scheduled for cardioversion, D-Dimer levels were also decreased by 32.3% (compared to VKA with 37.6%) [54]. In addition, a clinical study conducted by Spyropoulos et al. [71] reported D-dimer levels remaining consistently below the normal cut-off in rivaroxaban-treated patients with venous thromboembolism, while high D-Dimer levels could support the identification of elevated VTE risks in medically ill patients [71]. Also, as shown in the COMMANDER HF trial, D-dimer aided to predict stroke risk and rivaroxaban benefit in a HF patient population [72].
Platelet activation
Platelet activation can lead to adhesion and aggregation through platelet receptors. The activation of platelets is a key event during blood clotting and is highly interlinked with the coagulation cascade. During clotting, thrombin can induce activation of PAR-1 and PAR-4, leading to downstream signaling events such as granule secretion, and studies suggest that glycoprotein Ib-IX receptor complex signaling cooperates with PAR signaling to promote platelet activation in response to low thrombin concentrations [73]. Platelets release the contents of granules, including β-thromboglobulin, P-selectin and von Willebrand factor (vWF) [34]. It has been hypothesized that rivaroxaban may affect the platelet activation process, and concentrations of β-thromboglobulin, thrombospondin, vWF and P-selectin have been used as potential biomarkers to address this hypothesis [74, 75].
In an in vitro study in which blood was spiked with rivaroxaban, there was a reduction in P-selectin surface expression, indicating a modest attenuation of thrombin-induced or TF-induced activation of platelets. An additional finding in this study was that the addition of rivaroxaban to blood before adenosine diphosphate (ADP)-induced activation led to limited but consistent attenuation of activation. The authors concluded that these results merit further investigation but suggest that rivaroxaban could interfere directly with ADP-induced platelet activation [76].
Building on the evidence reported in preclinical studies, similar trends for platelet activation biomarkers have been reported in the clinical setting. For example, in a proteomic analysis of rivaroxaban-treated patients with nonvalvular AF, there were significant decreases (p = 0.0246) in circulating P-selectin from day 1 to day 24 of treatment [25]. Furthermore, Weisshaar et al. [50] reported a trial in healthy individuals randomized to treatment with rivaroxaban. In these participants, concentrations of β-thromboglobulin in shed blood were significantly decreased compared to pre-dose concentrations (1534 IU/mL at baseline vs. 987 IU/mL at 3 h after the dose; p ≤ 0.001). Additionally, an analysis of rivaroxaban-treated patients with AF and LA/LAA thrombi reported a significant baseline-adjusted decrease in mean serum concentration of vWF from baseline to end of treatment (− 32%; p < 0.001) [53]. Also, Ordi-Ros et al. reported a decrease in the platelet activation biomarker over time (3 years) after treatment of patients with thrombotic Antiphospolipid Syndrome (APS) with Rivaroxaban, whereby vWF levels decrease only slightly. No significant differences were seen here compared to Warfarin treatment [77].
While a trend for reduction in the plasma levels of these platelet activation biomarkers has been reported across various studies, other clinical investigations have failed to find significant changes following rivaroxaban treatment [34, 70, 78]. Specifically, Steppich et al. [34] reported a clinical study of patients with nonvalvular AF and found no significant reductions from baseline in the plasma levels of β-thromboglobulin, thrombospondin, vWF or P-selectin after treatment with rivaroxaban [34]. Similarly, in another clinical study of 10 rivaroxaban-treated (and 17 dabigatran-treated) patients with nonvalvular AF, Zemer-Wassercug et al. [78] reported no significant differences between baseline and post-rivaroxaban treatment platelet reactivity as measured by the proportion of P-selectin expressed on the platelet membrane [78].
Inflammatory activity
Inflammatory processes are closely linked with the blood coagulation cascade. For example, thrombin can interact with PAR-1 on endothelial cells, fibroblasts and monocytes, causing a downstream signaling cascade that triggers production of pro-inflammatory molecules, including monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) [79,80,81,82]. In addition, PAR-2, expressed by vascular endothelial cells, was found to be involved in thrombin-independent, pro-inflammatory signaling, through interaction with TF and FVIIa [62, 63]. Several studies have investigated pro-inflammatory molecules as potential biomarkers of inflammatory changes following rivaroxaban treatment.
Tissue explant experiments have reported reductions in the levels of mRNA expression relating to inflammatory molecules in rivaroxaban-exposed samples compared with controls, including IL-6 [41, 83, 84], MCP-1 [59, 83, 85, 86], IL-1β [59, 83] and TNF-α [41, 59], and in protein concentrations of IL-6 [87]. However, not all results have shown this trend and an investigation of the cytokines released from monocytes during the process of thrombin generation found that rivaroxaban had no significant influence on IL-6 or TNF-α secretion [88].
In addition to tissue explant studies, investigations using inflammatory animal models have reported reductions in the levels of mRNA expression and plasma concentrations of various molecules in rivaroxaban-treated animals compared with controls, including IL-6 [89,90,91,92], MCP-1 [89], IL-1β [58] and TNF-α [58, 59, 89]. Terry et al. [93] investigated the effects of rivaroxaban treatment on pro-inflammatory molecule levels in a mouse model of catheter thrombosis. This study reported lower concentrations of MCP-1 protein in rivaroxaban-treated mice than in controls, but plasma levels of IL-6 and TNF-α did not significantly differ between groups [93].
The evidence of anti-inflammatory effects of rivaroxaban in the clinical setting is limited; however, some clinical investigations have provided hints of an association between rivaroxaban treatment and inflammatory processes. For example, in the X-TRA study, elevated levels of high sensitivity IL-6 (hsIL-6) at baseline were significantly associated with thrombus reduction or resolution in rivaroxaban-treated patients with AF (odds ratio, 4.909; p = 0.021). However, in this study, rivaroxaban treatment did not lead to significant changes in concentrations of hsIL-6 between baseline and end of rivaroxaban treatment even though the patients in general benefited from rivaroxaban treatment [53]. In the X-VeRT study, reductions of 12.5% and 9.2% for hs-CRP and hs-IL-6 respectively were observed [54]. Similarly, in another study of patients with AF, no significant changes from baseline in blood levels of TNF-α or IL-6 were reported after 6 months of rivaroxaban treatment [26].
Endothelial changes
In addition to their role in coagulation, thrombin and FXa elicit multiple effects on endothelial cells, including the modulation of the expression of genes encoding proteins that play a role in adhesion and inflammation either directly by PAR receptor signaling on the endothelial surface or indirectly via PAR-initiated platelet activation and subsequent adhesion once endothelium is damaged and aggregation during blood clot formation [94].
To investigate how rivaroxaban-induced inhibition of FXa and thrombin generation could impact endothelial cells, in addition to the levels of excreted inflammatory biomarkers, biomarkers of endothelial surface activation have been investigated. Cell surface molecules that have been studied include: thrombomodulin, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and other extracellular markers such as matrix metalloproteinase (MMP)-9. Thrombomodulin is a protein expressed on endothelial cell surfaces which can bind thrombin, subsequently activate protein C, and inhibit the process of coagulation [12]. ICAM-1 and VCAM-1 are surface adhesion molecules expressed on endothelial cells [85] and MMP-9 is a zinc-dependent enzyme involved in degradation of the extracellular matrix during biological processes. Raised concentrations of MMP-9 protein have been associated with various disease states, including cardiovascular conditions [95, 96].
In in vitro studies the downregulation of ICAM-1 and VCAM-1 mRNA expression following administration of rivaroxaban compared with untreated controls has been reported [59, 83, 85]. In agreement to these data, animal studies have shown reductions in the expression level of ICAM-1 mRNA in the rivaroxaban group compared to control animals [58]. Similarly, preclinical studies have suggested that MMP-9 could be a potential biomarker of the inhibition of endothelial activation by rivaroxaban. In animal models, the expression of MMP-9 was reduced in rivaroxaban-treated animals compared with controls [59, 93]. In addition, in a study conducted by Monux et al. (2017), in vitro incubation of rivaroxaban with samples of human abdominal aortic aneurysm sites with intraluminal thrombus resulted in significantly reduced MMP-9 expression to levels similar to those found in control aortas [87]. However, in contrast, Rosenkranz et al. [87] reported no significant effect on MMP-2 and MMP-9 levels in clot-stimulated, vascular smooth muscle cells treated with rivaroxaban [41].
In line with the known antithrombotic role of thrombomodulin, a clinical study in 23 patients with nonvalvular AF reported a significant increase in mean concentrations of plasma thrombomodulin after 6 months of treatment with rivaroxaban (2.9 fibrinolytic units (FU)/mL to 3.2 FU/mL; p = 0.003) [26]. In a proteomic analysis of the ROCKET-AF trial, a randomized study of patients with nonvalvular AF receiving rivaroxaban or warfarin treatment, investigators reported upregulation of soluble thrombomodulin during treatment with rivaroxaban compared with treatment with warfarin [25]. This study also reported a significant reduction (p = 0.0338) in MMP-9 protein concentrations from baseline to week 24 of rivaroxaban treatment. There was additionally a trend toward a greater decrease in MMP-9 levels in rivaroxaban-treated patients than in warfarin-treated patients [25]. Although these trials provide promising data for a relationship between rivaroxaban treatment and molecular biomarkers of endothelial changes, further clinical studies will be required to confirm these findings.
In an in vitro experiment for diabetic endothelial senescence, HUVECs were cultured with/without rivaroxaban under high glucose (HG). Senescence- associated-β-galactosidase (SA-β-gal), p53, p21, and p16INK4a were increased by HG via PAR receptors and restored by rivaroxaban, which restored telomerase activity and preserved telomere length, as well, suppressed O2–, p22phox, and ICAM1 and restored NOx and eNOS. In dyslipidemic diabetic mice, plasma glucose, total cholesterol, and triglycerides were increased for 4 weeks but were not changed by rivaroxaban. However, rivaroxaban decreased SA-β-gal and telomerase and preserved telomere length in the aortic endothelium. Rivaroxaban activated eNOS, inhibited p22phox, increased plasma NOx, and decreased O2–. Thus, rivaroxaban prevented replicative senescence in HUVECs and aortic endothelial cells, restored endothelial function and prevented the progression of atherosclerosis [97]. A clinical study with type 2 diabetes mellitus and subclinical inflammation showed that rivaroxaban compared to Aspirin could improve endothelial function based different measures such as post-ischaemic forearm blood flow during reactive hyperaemia, skin blood flow, sP-Selectin or platelet-derived microparticles which stimulate endothelial repair [98].
Disease and organ biomarkers
For many cardiovascular diseases, including those that are indications for rivaroxaban, studies have suggested that disease states are associated with the in vivo levels of molecular biomarkers of coagulation and inflammation. Therefore, many of the molecular biomarkers discussed above could be important as biomarkers for disease severity and progression. Table 2 summarizes some examples of studies that have reported associations between such molecular biomarkers of coagulation and inflammation in patients with cardiovascular disease [19, 99,100,101,102,103,104,105,106,107,108].
In addition to the molecular biomarkers of fibrin formation and coagulation, platelet activation, inflammation and endothelial changes discussed above, changes to concentrations of other molecular biomarkers have been found to be associated with specific disease states. As such, exploratory studies have investigated the potential effects of rivaroxaban exposure on levels of a variety of disease biomarkers.
Studies have suggested that AF is associated with systemic and cardiac oxidation and shares many of the same risk factors as atherosclerosis, a disease that is perpetuated by oxidative stress [111]. Preclinical studies have reported mixed results for the investigations of the effects of rivaroxaban on known markers of oxidative stress, such as malonaldehyde, reactive oxygen species, nitric oxide synthase isotype 2 and nitrogen oxide [87, 112, 113]. For example, in a rat model of peripheral-ischemia reperfusion, animals treated with rivaroxaban had significantly lower (p < 0.05) mean plasma levels of malondialdehyde (24.9 µmol/L) than sham mice (75.6 µmol/L). However, there were no significant differences between groups in plasma levels of nitrogen oxide [112].
An enhanced renin-angiotensin system causes hypertension, an important risk factor for chronic kidney disease. Treatment with rivaroxaban decreased the urinary albumin excretion and attenuated histologic changes of glomerular hypertrophy, mesangial matrix expansion, effacement of the podocyte foot process, and thickened glomerular basement membrane in hypertensive mice overexpressing renin. A renal protective effect of rivaroxaban provides an important clinical implication on the underlying mechanism by which rivaroxaban is associated with lower risks of decline in estimated glomerular filtration rate, doubling of serum creatinine, and acute kidney injury in patients with nonvalvular atrial fibrillation in clinical studies [114].
Finally, studies have investigated other biomarkers as a means of stratifying patients into groups that can gain the most benefit from rivaroxaban treatment. For example, in a post hoc analysis of the ATLAS ACS 2-TIMI 51 trial, patients with ACS were stratified by risk using biomarkers of high-risk disease [115]. Positive biomarker was predefined as either serum troponin concentration above the decision limit or serum creatine kinase–myocardial band isozyme above the upper limit, at normal level, or both. Based on the efficacy results, it was concluded that biomarker-positive patients with no previous history of stroke or transient ischemic attack may be an optimal target population to receive rivaroxaban in combination with antiplatelet therapy for secondary prevention of ACS. A recent study testing the efficacy of rivaroxaban versus aspirin for secondary stroke prevention in ESUS (Embolic Stroke of Undetermined Source) patients investigated whether hs-cTnT (high-sensitivity cardiac Troponin T) levels might be associated with major vascular events and if it may help to identify patients who would benefit from anticoagulation after ESUS (substudy of NAVIGATE-ESUS study). Here it was found that hs-cTnT is indeed associated with increased cardiovascular event rates which means that these biomarkers could support stratification of patients for cardiovascular risk, but not for decision -making regarding anticoagulant therapy [116]. Similarly, in the COMMANDER-HF trial, inclusion of patients in the study based on elevated plasma concentrations of natriuretic peptides (NPs) as selection criteria with the goal to support HF ascertainment and risk enrichment was performed. The results showed that elevated NPs for inclusion increased event rates allowing earlier completion of the trial but did not modify treatment effect with rivaroxaban [117]. In the VaLiDate-R study which started in January 2019 and is still ongoing at time of preparation of this manuscript, impaired endogenous fibrinolysis is assessed as potential novel biomarker for risk-stratification to identify patients who would benefit from more potent antithrombotic therapy [118].
Outlook and novel approaches
In recent years, there has been an increase in the number of investigations using biomarkers to probe various biological processes, and future technical advances may contribute to further growth. For example, proteomic profiling techniques using mass spectrometry or affinity multiplexing assays could accelerate novel biomarker discovery. Proteomics studies can evaluate all proteins in a system, making them hypothesis-free and unbiased [119, 120]. A consensus statement on outcome parameters in AF trials has highlighted plasma proteomics as a potentially useful approach to identify novel drug effects in early phases of trials. This technique could be used to identify surrogates for understanding the pathophysiologic mechanisms underlying a given disease [121].
The potential of such proteomic studies is highlighted by results from recent investigations. For example, a study among participants from the Framingham Heart Study Offspring Cohort identified eight proteins associated with risk of incident AF after adjustment for age and sex. However, authors noted that further investigation would be required to confirm if any of these markers are mechanistically related to AF development [122]. In addition, a recent study tested a pragmatic biomarker discovery strategy that integrated automated clinical biobanking (using electronic health records) and proteomics. This study identified two potential biomarkers that robustly predicted HF across diverse clinical settings [123].
Further biomarker investigations are currently ongoing in samples of the COMPASS trial in which the efficacy of dual pathway inhibition with 2.5 mg twice daily rivaroxaban and aspirin in patients with coronary artery disease or peripheral arterial disease or both was demonstrated. Here, platelet aggregation, platelet activation and inflammation markers, thrombin generation kinetics and tissue factor-induced platelet–fibrin clot strength will be measured at baseline, and 4 and 12 weeks after randomization in order to evaluate if treatment with rivaroxaban is associated with a reduction in platelet activation and aggregation, inflammation and coagulation markers [124].
Conclusion
Based on the studies identified and discussed in this review, there are several results that support the hypothesis that rivaroxaban has effects that extend beyond the coagulation pathway. In our review of preclinical and clinical studies, which tested the impact of rivaroxaban, we found several publications that reported results on biomarkers involved in various biological pathways and processes, such as biomarkers of coagulation status, including direct target engagement biomarkers (F1 + 2 and TAT complex) and indirect biomarkers (D-dimers).
In addition, and probably due to the interaction of the coagulation system with other biological pathways, our review identified various studies that have reported impacts of rivaroxaban on biomarkers of platelet effects, inflammation and endothelial changes. We found that the evidence for an effect of rivaroxaban on biomarkers of platelet activity, endothelial function, inflammation and oxidative stress derives predominantly from preclinical studies. Such results are useful for hypothesis generation but may not always match the human situation and consequently not translate into clinics. Therefore, additional animal studies and -most of all- clinical studies would be required.
Investigations of molecular biomarkers have limitations: There have been inconsistencies in the definitions used for different types of biomarkers. To address this problem, the FDA–National Institutes of Health working group developed the Biomarkers, EndpointS, and other Tools (BEST) resource in 2018, to provide guidance on the terminology for this type of research [8]. Another issue is the use of different types of assays for one analyte and of the results for inter-study comparisons – often with inconsistencies and lack of standardization between assays. A representative and well-investigated example are the high inter-laboratory and inter-method variabilities in D-dimer assays, likely due to the use of different reagents and the lack of standardized internationally certified calibrators and quality-control measures [125, 126]. Furthermore, depending on the state of the disease at the time of the blood draw, the results of the different biomarkers may vary which has to be considered and can limit their applicability for direct risk stratification in clinical practice. Translation to subsequent use as potential surrogate biomarkers requires further clinical validation investigations [8, 13].
In conclusion, the effect of rivaroxaban on D-dimers, TAT complex and F1 + 2 as well as inflammatory markers could be shown in clinical studies. Preclinical and clinical studies have also provided data, which showed effects of rivaroxaban on the pathways of platelet activation and endothelial changes. Future investigations will be required to further manifest these findings and potentially apply them in clinical situations.
Data availability
Not applicable.
Abbreviations
- ACS:
-
Acute coronary syndrome
- ADP:
-
Adenosine diphosphate
- AF:
-
Atrial fibrillation
- BEST:
-
Biomarkers, EndpointS, and other Tools
- DVT:
-
Deep vein thrombosis
- F1 + 2:
-
Prothrombin fragment 1 + 2
- FDA:
-
US Food and Drug Administration
- FU:
-
Fibrinolytic units
- FVIIa:
-
Factor VIIa
- FX:
-
Factor X
- FXa:
-
Activated factor X
- HF:
-
Heart failure
- hsIL-6:
-
High sensitivity interleukin-6
- ICAM-1:
-
Intercellular adhesion molecule-1
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- LA/LAA:
-
Left atrial/left atrial appendage
- MCP-1:
-
Monocyte chemoattractant protein-1
- MMP:
-
Matrix metalloproteinase
- PAR:
-
Protease-activated receptor
- PCI:
-
Percutaneous coronary intervention
- PE:
-
Pulmonary embolism
- TAT:
-
Thrombin–antithrombin
- TF:
-
Tissue factor
- β-ThG:
-
Thromboglobulin
- TNF-α:
-
Tumor necrosis factor-α
- VCAM-1:
-
Vascular cell adhesion molecule-1
- vWF:
-
Von Willebrand factor
References
Rasche H (2001) Haemostasis and thrombosis: an overview. Eur Heart J Suppl. 3((suppl_Q)):Q3-7
Fisher WD (2011) Impact of venous thromboembolism on clinical management and therapy after hip and knee arthroplasty. Can J Surg 54(5):344–351
Watson T, Shantsila E, Lip GY (2009) Mechanisms of thrombogenesis in atrial fibrillation: virchow’s triad revisited. Lancet 373(9658):155–166
Pop C, Matei C, Petris A (2019) Anticoagulation in acute coronary syndrome: review of major therapeutic advances. Am J Ther 26(2):e184–e197
Thomas DP, Roberts HR (1997) Hypercoagulability in venous and arterial thrombosis. Ann Intern Med 126(8):638–644
Kubitza D et al (2005) Safety, pharmacodynamics, and pharmacokinetics of BAY 59–7939 – an oral, direct Factor Xa inhibitor – after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 61(12):873–880
Palta S, Saroa R, Palta A (2014) Overview of the coagulation system. Indian J Anaesth 58(5):515–523
FDA-NIH Biomarker Working Group, BEST (Biomarkers, EndpointS, and other Tools) Resource. 2016. https://www.ncbi.nlm.nih.gov/books/NBK326791/?report=classic. Accessed 11 Feb 2019.
Eikelboom JW et al (2017) Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 377(14):1319–1330
Borst O et al (2018) Inhibitory mechanisms of very low-dose rivaroxaban in non-ST-elevation myocardial infarction. Blood Adv 2(6):715–730
Göbel K et al (2018) The coagulation factors fibrinogen, thrombin, and factor XII in inflammatory disorders—a systematic review. Front Immunol. https://doi.org/10.3389/fimmu.2018.01731
Esmon CT (2014) Targeting factor Xa and thrombin: impact on coagulation and beyond. Thromb Haemost 111(4):625–633
Bai JPF et al (2011) Translational biomarkers: from preclinical to clinical a report of 2009 AAPS/ACCP Biomarker Workshop. AAPS J 13(2):274–283
Samama MM (2011) The mechanism of action of rivaroxaban – an oral, direct Factor Xa inhibitor–compared with other anticoagulants. Thromb Res 127(6):497–504
Perzborn E et al (2010) Rivaroxaban: a new oral factor Xa inhibitor. Arterioscler Thromb Vasc Biol 30(3):376–381
Perzborn E et al (2005) In vitro and in vivo studies of the novel antithrombotic agent BAY 59–7939—an oral, direct Factor Xa inhibitor. J Thromb Haemost 3(3):514–521
Arachchillage DR et al (2015) Rivaroxaban and warfarin achieve effective anticoagulation, as assessed by inhibition of TG and in-vivo markers of coagulation activation, in patients with venous thromboembolism. Thromb Res 135(2):388–393
Ebner M et al (2017) Emergency coagulation assessment during treatment with direct oral anticoagulants: limitations and solutions. Stroke 48(9):2457–2463
Gheorghiade M et al (2011) Pharmacokinetics and pharmacodynamics of rivaroxaban and its effect on biomarkers of hypercoagulability in patients with chronic heart failure. J Heart Lung Transplant 30(2):218–226
Graff J et al (2007) Effects of the oral, direct factor Xa inhibitor rivaroxaban on platelet-induced thrombin generation and prothrombinase activity. J Clin Pharmacol 47(11):1398–1407
Green L et al (2010) The impact of elective knee/hip replacement surgery and thromboprophylaxis with rivaroxaban or dalteparin on thrombin generation. Br J Haematol 151(5):469–476
Hagii J et al (2016) Effect of rivaroxaban on prothrombin fragment 1+2 compared with warfarin in patients with acute cardioembolic stroke: insight from its serial measurement. Thromb Res 148:9–14
Helin TA et al (2017) Effects of thromboprophylactic doses of apixaban and rivaroxaban on coagulation and thrombin generation in association with total hip replacement. J Thromb Thrombolysis 43(4):562–569
Hitaka Y et al (2016) Circadian variations in laboratory measurements of coagulation assays after administration of rivaroxaban or warfarin in patients with nonvalvular atrial fibrillation. J Cardiol 68(6):529–535
Chan MY et al (2012) Plasma proteomics of patients with non-valvular atrial fibrillation on chronic anti-coagulation with warfarin or a direct factor Xa inhibitor. Thromb Haemost 108(6):1180–1191
Katoh H, Nozue T, Michishita I (2017) Anti-inflammatory effect of factor-Xa inhibitors in Japanese patients with atrial fibrillation. Heart Vessels 32(9):1130–1136
Kitagawa F et al (2017) Assessment of trough rivaroxaban concentrations on markers of coagulation activation in nonvalvular atrial fibrillation population. Heart Vessels 32(5):609–617
Mani H et al (2011) Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost 106(1):156–164
Molenaar PJ, Dinkelaar J, Leyte A (2012) Measuring rivaroxaban in a clinical laboratory setting, using common coagulation assays, Xa inhibition and thrombin generation. Clin Chem Lab Med 50(10):1799–1807
Nakano Y et al (2015) Clinical usefulness of measuring prothrombin time and soluble fibrin levels in Japanese patients with atrial fibrillation receiving rivaroxaban. J Cardiol 65(3):185–190
Oswald E et al (2015) Results of rotational thromboelastometry, coagulation activation markers and thrombin generation assays in orthopedic patients during thromboprophylaxis with rivaroxaban and enoxaparin: a prospective cohort study. Blood Coagul Fibrinolysis 26(2):136–144
Platton S, Bowles L, MacCallum P (2017) Effects of rivaroxaban on routine coagulation screening tests using commonly used reagents. Br J Haematol 179(3):511–513
Pratt J, Crispin P (2018) Screening test for direct oral anticoagulants with the dilute Russell viper venom time. Eur J Haematol 100(6):567–574
Steppich B et al (2017) Effect of the FXa inhibitors rivaroxaban and apixaban on platelet activation in patients with atrial fibrillation. J Thromb Thrombolysis 43(4):490–497
Suzuki S et al (2018) An analysis on distribution and inter-relationships of biomarkers under rivaroxaban in Japanese patients with non-valvular atrial fibrillation (CVI ARO 1). Drug Metab Pharmacokinet 33(4):188–193
Tajiri K et al (2015) Impact of rivaroxaban compared with warfarin on the coagulation status in Japanese patients with non-valvular atrial fibrillation: a preliminary analysis of the prothrombin fragment 1+2 levels. J Cardiol 65(3):191–196
Wan H et al (2016) An in-vitro evaluation of direct thrombin inhibitor and factor Xa inhibitor on tissue factor-induced thrombin generation and platelet aggregation: a comparison of dabigatran and rivaroxaban. Blood Coagul Fibrinolysis 27(8):882–885
Vranckx P et al (2015) Peri-procedural use of rivaroxaban in elective percutaneous coronary intervention to treat stable coronary artery disease the X-PLORER trial. Thromb Haemost 114(2):258–267
Douxfils J et al (2012) Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res 130(6):956–966
Parry TJ et al (2011) Arterial antithrombotic activity of rivaroxaban, an orally active factor Xa inhibitor, in a rat electrolytic carotid artery injury model of thrombosis. Blood Coagul Fibrinolysis 22(8):720–726
Rosenkranz AC, Schror K, Rauch BH (2011) Direct inhibitors of thrombin and factor Xa attenuate clot-induced mitogenesis and inflammatory gene expression in human vascular smooth muscle cells. Thromb Haemost 106(3):561–562
Sparkenbaugh EM et al (2014) Differential contribution of FXa and thrombin to vascular inflammation in a mouse model of sickle cell disease. Blood 123(11):1747–1756
Lim HY et al (2019) A review of global coagulation assays - Is there a role in thrombosis risk prediction? Thromb Res 179:45–55
Hirota N et al (2020) Analysis of bioMARKer distribution and individual reproducibility under rivaroxaban treatment in japanese patients with non-valvular atrial fibrillation (R-MARK Study, CVI ARO2). Int Heart J 61(4):695–704
Biemond BJ et al (2007) Prevention and treatment of experimental thrombosis in rabbits with rivaroxaban (BAY 597939)–an oral, direct factor Xa inhibitor. Thromb Haemost 97(3):471–477
Zhou W et al (2013) Hemostatic therapy in experimental intracerebral hemorrhage associated with rivaroxaban. Stroke 44(3):771–778
Gosselin RC et al (2014) Comparison of anti-Xa and dilute Russell viper venom time assays in quantifying drug levels in patients on therapeutic doses of rivaroxaban. Arch Pathol Lab Med 138(12):1680–1684
Aronson DL et al (1977) Generation of the combined prothrombin activation peptide (F1–2) during the clotting of blood and plasma. J Clin Invest 60(6):1410–1418
Ueno EI et al (2020) Monitoring the roles of prothrombin activation fragment 1 and 2 (F1 + 2) in patients with atrial fibrillation receiving rivaroxaban and apixaban. J Thromb Thrombolysis 50(2):371–379
Weisshaar S et al (2014) Antithrombotic triple therapy and coagulation activation at the site of thrombus formation: a randomized trial in healthy subjects. J Thromb Haemost 12(11):1850–1860
Brunner-Ziegler S et al (2016) Comparison between the impact of morning and evening doses of rivaroxaban on the circadian endogenous coagulation rhythm in healthy subjects. J Thromb Haemost 14(2):316–323
Liles J et al (2016) Increased level of thrombotic biomarkers in patients with atrial fibrillation despite traditional and new anticoagulant therapy. Clin Appl Thromb Hemost 22(8):743–748
Miyazawa K et al (2018) Left atrial thrombus resolution in non-valvular atrial fibrillation or flutter: biomarker substudy results from a prospective study with rivaroxaban (X-TRA). Ann Med 50:511–518
Kirchhof P et al (2020) Effects of rivaroxaban on biomarkers of coagulation and inflammation: a post hoc analysis of the X-VeRT trial. TH Open 4(1):e20–e32
Hoek JA et al (1988) Laboratory and clinical evaluation of an assay of thrombin-antithrombin III complexes in plasma. Clin Chem 34(10):2058–2062
Stepien E et al (2007) Factors influencing thrombin generation measured as thrombin-antithrombin complexes levels and using calibrated automated thrombogram in patients with advanced coronary artery disease. Pol Arch Med Wewn 117(7):297–305
Deguchi K et al (1991) Dynamic fluctuations in blood of thrombin/antithrombin III complex (TAT). Am J Hematol 38(2):86–89
Dittmeier M et al (2016) Pretreatment with rivaroxaban attenuates stroke severity in rats by a dual antithrombotic and anti-inflammatory mechanism. Thromb Haemost 115(4):835–843
Hara T et al (2015) Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis 242(2):639–646
Coughlin SR (2005) Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 3(8):1800–1814
Posma JJ et al (2019) Roles of coagulation proteases and PARs (Protease-Activated Receptors) in mouse models of inflammatory diseases. Arterioscler Thromb Vasc Biol 39(1):13–24
Camerer E, Huang W, Coughlin SR (2000) Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA 97(10):5255–5260
Demetz G et al (2010) Tissue Factor-Factor VIIa complex induces cytokine expression in coronary artery smooth muscle cells. Atherosclerosis 212(2):466–471
Chung CC et al (2018) Factor Xa inhibition by rivaroxaban regulates fibrogenesis in human atrial fibroblasts with modulation of nitric oxide synthesis and calcium homeostasis. J Mol Cell Cardiol 123:128–138
Puech C et al (2019) Direct oral anticoagulants are associated with limited damage of endothelial cells of the blood-brain barrier mediated by the thrombin/PAR-1 pathway. Brain Res 1719:57–63
Petzold T et al (2020) Rivaroxaban Reduces arterial thrombosis by inhibition of FXa-driven platelet activation via protease activated receptor-1. Circ Res 126(4):486–500
Gaffney PJ, Joe F (1979) The lysis of crosslinked human fibrin by plasmin yields initially a single molecular complex D dimer-E. Thromb Res 15(5–6):673–687
Soomro AY et al (2016) The current role and future prospects of D-dimer biomarker. Eur Heart J Cardiovasc Pharmacother 2(3):175–184
AlKhalfan F et al (2018) D-Dimer levels and effect of rivaroxaban on those levels and outcomes in patients with acute coronary syndrome (an ATLAS ACS-TIMI 46 trial substudy). Am J Cardiol 122(9):1459–1464
Scheres LJJ et al (2018) Measurement of coagulation factors during rivaroxaban and apixaban treatment: results from two crossover trials. Res Pract Thromb Haemost 2(4):689–695
Spyropoulos AC et al (2020) Modified IMPROVE VTE risk score and elevated D-dimer identify a High venous thromboembolism risk in acutely ill medical population for extended thromboprophylaxis. TH Open 4(1):e59–e65
Ferreira JP et al (2020) Plasma D-dimer concentrations predicting stroke risk and rivaroxaban benefit in patients with heart failure and sinus rhythm: an analysis from the COMMANDER-HF trial. Eur J Heart Fail 23:648–656
Estevez B et al (2016) Signaling-mediated cooperativity between glycoprotein Ib-IX and protease-activated receptors in thrombin-induced platelet activation. Blood 127(5):626–636
Flierl U et al (2013) The direct factor Xa inhibitor Rivaroxaban reduces platelet activation in congestive heart failure. Pharmacol Res 74:49–55
Pignatelli P et al (2016) Anti Xa oral anticoagulants inhibit in vivo platelet activation by modulating glycoprotein VI shedding. Pharmacol Res 113(Pt A):484–489
Ringwala SM, Dibattiste PM, Schneider DJ (2012) Effects on platelet function of a direct acting antagonist of coagulation factor Xa. J Thromb Thrombolysis 34(3):291–296
Ordi-Ros J et al (2019) Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med 171(10):685–694
Zemer-Wassercug N et al (2015) The effect of dabigatran and rivaroxaban on platelet reactivity and inflammatory markers. J Thromb Thrombolysis 40(3):340–346
Sower LE et al (1995) Thrombin induces IL-6 production in fibroblasts and epithelial cells. Evidence for the involvement of the seven-transmembrane domain (STD) receptor for alpha-thrombin. J Immunol 155(2):895–901
Chen D et al (2008) Protease-activated receptor 1 activation is necessary for monocyte chemoattractant protein 1-dependent leukocyte recruitment in vivo. J Exp Med 205(8):1739–1746
Yang H et al (2013) Induction of tumor necrosis factor (TNF) release from subtypes of T cells by agonists of proteinase activated receptors. Mediators Inflamm 2013:165453
Foley JH, Conway EM (2016) Cross talk pathways between coagulation and inflammation. Circ Res 118(9):1392–1408
Sanada F et al (2016) Activated factor X induces endothelial cell senescence through IGFBP-5. Sci Rep 6:35580
Wypasek E et al (2020) Effects of rivaroxaban and dabigatran on local expression of coagulation and inflammatory factors within human aortic stenotic valves. Vascul Pharmacol 130:106679
Ellinghaus P et al (2016) Expression of pro-inflammatory genes in human endothelial cells: comparison of rivaroxaban and dabigatran. Thromb Res 142:44–51
Ishibashi Y, Matsui T, Fukami K, Ueda S, Okuda S, Yamagishi S (2015) Rivaroxaban inhibits oxidative and inflammatory reactions in advanced glycation end product-exposed tubular cells by blocking thrombin/protease-activated receptor-2 system. Thromb Res 135(4):770–3
Monux G et al (2017) FXa inhibition by rivaroxaban modifies mechanisms associated with the pathogenesis of human abdominal aortic aneurysms. Br J Clin Pharmacol 83(12):2661–2670
Laurent M et al (2014) Comparative study of the effect of rivaroxaban and fondaparinux on monocyte’s coagulant activity and cytokine release. Exp Hematol Oncol 3(1):30
Zhou Q et al (2011) Evaluation of plaque stability of advanced atherosclerotic lesions in apo E-deficient mice after treatment with the oral factor Xa inhibitor rivaroxaban. Mediators Inflamm 2011:432080
Goto M et al (2016) Rivaroxaban, a factor Xa inhibitor, induces the secondary prevention of cardiovascular events after myocardial ischemia reperfusion injury in mice. Int J Cardiol 220:602–607
Daci A et al (2020) Rivaroxaban improves vascular response in LPS-induced acute inflammation in experimental models. PLoS One 15(12):e0240669
Ding Y et al (2020) Factor Xa inhibitor rivaroxaban suppresses experimental abdominal aortic aneurysm progression via attenuating aortic inflammation. Vascul Pharmacol 136:106818
Terry CM, He Y, Cheung AK (2016) Rivaroxaban improves patency and decreases inflammation in a mouse model of catheter thrombosis. Thromb Res 144:106–112
Bergmeier W, Hynes RO (2011) Extracellular matrix proteins in hemostasis and thrombosis. Cold Spring Harb Perspect in Biol 4(2):a005132
Gerlach RF et al (2005) Effect of anticoagulants on the determination of plasma matrix metalloproteinase (MMP)-2 and MMP-9 activities. Anal Biochem 344(1):147–149
Halade GV, Jin YF, Lindsey ML (2013) Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 139(1):32–40
Maeda M, Tsuboi T, Hayashi T (2019) An inhibitor of activated blood coagulation factor x shows anti-endothelial senescence and anti-atherosclerotic effects. J Vasc Res 56(4):181–190
Pistrosch F et al (2021) Rivaroxaban compared with low-dose aspirin in individuals with type 2 diabetes and high cardiovascular risk: a randomised trial to assess effects on endothelial function, platelet activation and vascular biomarkers. Diabetologia 64(12):2701–2712
Conway DS et al (2004) Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol 43(11):2075–2082
Wu N et al (2013) Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol 169(1):62–72
Li J et al (2010) Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm 7(4):438–444
Olson NC et al (2014) Inflammation markers and incident venous thromboembolism: the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. J Thromb Haemost 12(12):1993–2001
Matos MF et al (2011) The role of IL-6, IL-8 and MCP-1 and their promoter polymorphisms IL-6 -174GC, IL-8 -251AT and MCP-1 -2518AG in the risk of venous thromboembolism: a case-control study. Thromb Res 128(3):216–220
Wells PS et al (2003) Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 349(13):1227–1235
Wu CT et al (2018) Plasma D-dimer is not useful in the prediction of deep vein thrombosis after total knee arthroplasty in patients using rivaroxaban for thromboprophylaxis. J Orthop Surg Res 13(1):173
Cohen AT et al (2014) D-dimer as a predictor of venous thromboembolism in acutely ill, hospitalized patients: a subanalysis of the randomized controlled MAGELLAN trial. J Thromb Haemost 12(4):479–487
Borris LC et al (2008) Differences in urinary prothrombin fragment 1 + 2 levels after total hip replacement in relation to venous thromboembolism and bleeding events. J Thromb Haemost 6(10):1671–1679
Nymo SH et al (2014) Inflammatory cytokines in chronic heart failure: interleukin-8 is associated with adverse outcome. Results from CORONA. Eur J Heart Fail 16(1):68–75
Martins GL et al (2020) Comparison of inflammatory mediators in patients with atrial fibrillation using warfarin or rivaroxaban. Front Cardiovasc Med 7:114
Di Lullo L et al (2021) New evidence of direct oral anticoagulation therapy on cardiac valve calcifications, renal preservation and inflammatory modulation. Int J Cardiol 345:90–97
Yang K-C, Dudley SC (2013) Oxidative stress and atrial fibrillation. Circulation 128(16):1724–1726
Caliskan A et al (2014) Factor-Xa inhibitors protect against systemic oxidant damage induced by peripheral-ischemia reperfusion. J Thromb Thrombolysis 37(4):464–468
Richards GA et al (2018) The effects of dabigatran and rivaroxaban on markers of polymorphonuclear leukocyte activation. Pharmaceuticals (Basel) 11(2):46
Ichikawa H et al (2019) Rivaroxaban, a Direct factor xa inhibitor, ameliorates hypertensive renal damage through inhibition of the inflammatory response mediated by protease-activated receptor pathway. J Am Heart Assoc 8(8):e012195
Korjian S et al (2019) Safety and efficacy of rivaroxaban for the secondary prevention following acute coronary syndromes among biomarker-positive patients: Insights from the ATLAS ACS 2-TIMI 51 trial. Eur Heart J Acute Cardiovasc Care 8(2):186–193
Scheitz JF et al (2020) High-sensitivity cardiac troponin T for risk stratification in patients with embolic stroke of undetermined source. Stroke 51(8):2386–2394
Cunningham JW et al (2020) Natriuretic Peptide-based inclusion criteria in a heart failure clinical trial: insights from COMMANDER HF. JACC Heart Fail 8(5):359–368
Gue YX et al (2020) Rationale and design of “Can Very Low Dose Rivaroxaban (VLDR) in addition to dual antiplatelet therapy improve thrombotic status in acute coronary syndrome (VaLiDate-R)” study : a randomised trial modulating endogenous fibrinolysis in patients with acute coronary syndrome. J Thromb Thrombolysis 49(2):192–198
Geyer PE et al (2017) Revisiting biomarker discovery by plasma proteomics. Mol Syst Biol 13(9):942
Hathout Y (2015) Proteomic methods for biomarker discovery and validation. Are we there yet? Expert Rev Proteomics 12(4):329–31
Kirchhof P et al (2007) Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace 9(11):1006–1023
Ko D et al (2019) Proteomics profiling and risk of new-onset atrial fibrillation: framingham heart study. J Am Heart Assoc 8(6):e010976–e010976
Wells QS et al (2019) Accelerating biomarker discovery through electronic health records, automated biobanking, and proteomics. J Am Coll Cardiol 73(17):2195–2205
Tantry U et al (2020) Synergistic influence of rivaroxaban on inflammation and coagulation biomarkers in patients with coronary artery disease and peripheral artery disease on aspirin therapy. Future Cardiol 16(2):69–75
Riley RS et al (2016) Widely used types and clinical applications of D-dimer assay. Lab Med 47(2):90–102
Thachil J, Lippi G, Favaloro EJ (2017) D-dimer testing: laboratory aspects and current issues. Methods Mol Biol 1646:91–104
Acknowledgements
Medical writing support was provided by Kate Ward from Oxford PharmaGenesis Ltd, Oxford, UK.
Funding
Medical writing support was partially provided by Oxford PharmaGenesis™ Ltd, Oxford, UK and funded by Bayer AG, Berlin, Germany.
Author information
Authors and Affiliations
Contributions
SS, SS, SH, all authors contributed to the literature research, review and summary, and analyzed the publications regarding their relevance to preclinical and clinical studies of rivaroxaban and biomarkers of coagulation, inflammation, platelet activation, endothelial changes and oxidative activity. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors are employees of Bayer AG.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schiffer, S., Schwers, S. & Heitmeier, S. The effect of rivaroxaban on biomarkers in blood and plasma: a review of preclinical and clinical evidence. J Thromb Thrombolysis 55, 449–463 (2023). https://doi.org/10.1007/s11239-023-02776-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02776-z