Abstract
Arterial and venous thromboembolism is a major medical concern that requires therapeutic anticoagulation in various medical fields to prevent its drastic consequences. Despite significant advances in anticoagulant therapy, thrombosis remains a leading cause of morbidity and mortality worldwide. Traditional anticoagulants like heparin and vitamin K antagonists (VKAs) have shown efficacy in preventing and treating thrombosis but come with an inherent risk of bleeding due to their non-specific inhibition of multiple coagulation factors. Subsequent direct oral anticoagulants (DOACs), targeting specific factors such as Xa or thrombin, demonstrated improved safety profiles compared to VKAs, yet bleeding remains a concern. Accordingly, research is focused on developing anticoagulants with improved safety profiles. A safer class of anticoagulants would have broad appeal. The intrinsic pathway of coagulation, involving factor XI (FXI), has attracted attention as a potential target for safer anticoagulants. Preclinical studies and epidemiological data indicate that FXI deficiency or inhibition protects against thrombosis with minimal bleeding. Current research involves evaluating various FXI-directed strategies, and phase 2 studies have shown promising results in orthopedic surgery, atrial fibrillation, end-stage renal disease (ESRD), myocardial infarction, and ischemic stroke. Several agents, such as antisense oligonucleotides, monoclonal antibodies, small synthetic molecules, natural peptides, and aptamers, have been developed to inhibit FXI at different stages, offering potentially safer alternatives to traditional anticoagulants. However, the optimal balance between preventing thrombosis and the risk of bleeding associated with FXI inhibitors requires validation through extensive phase 3 clinical trials using definite clinical endpoints. Several of such trials are currently underway or planned to define the role of FXI inhibitors in clinical practice and determine the most suitable FXI inhibitor for each specific indication. The current review highlights the rationale behind developing FXI inhibitors, presenting the most advanced agents in development, summarizing completed clinical trials, and discussing ongoing research efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Structure
Factor XI (FXI), a 160-kDa glycoprotein serine protease, exists as a dimer with two identical subunits, each comprising 607 amino acids and linked by disulfide bonds. Each polypeptide is composed of a C-terminal light chain of 35 kDa, housing the trypsin-like catalytic domain, and an N-terminal heavy chain of 45 kDa, which contains four ~ 90 amino acid tandem repeats known as apple domains [1]. These apple domains play a crucial role in binding to various proteins; A1 interacts with high molecular weight kininogen (HK) and thrombin [2], A2 and A3 bind to FIX [3], A3 interacts with the platelet receptor GP1bα and heparin [4], and A4 binds to FXII and the other subunit of FXI [5, 6]. Activation of FXI occurs through cleavage by either XII or thrombin at the same site, leading to an intermediate structure termed 1/2FXIa, featuring one activated subunit [7]. Subsequently, FXIa activates FIX to FIXαβ by cleaving two activation sites [7]. While dimerization appears to be crucial for FXI activation, it is not a requirement for FIX cleavage, as monomeric FXI mutants can activate FIX at a similar rate as dimeric FXIa, albeit with reduced activation by FXII or thrombin [8] (Fig. 1).
Factor XI Structure and Function. Based on crystallographic structure analysis, FXI contains 4 "apple domains" shown as ribbons in gold, magenta, green, and blue, that form a disk-like structure with extensive interfaces at the base of the catalytic domain. The characterization of the apple disk structure, and its relationship to the catalytic domain, provide important insights into the binding of factor XI with substrates like factor IXa, thrombin and its interaction with platelets through the glycoprotein (GP) Ib receptor
Rationale behind targeting factor XI for anticoagulation therapy
Role of FXI in hemostasis
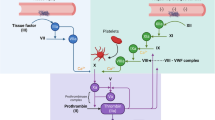
Hemostasis is initiated when tissue factor interacts with activated factor VII (TF-FVIIa) in what is called the tissue factor pathway. This interaction leads to the conversion of factor X into its active form, activated factor X (FXa). FXa then initiates a series of events that ultimately lead to the activation of factor II, leading to the formation of fibrin and the production of a hemostatic clot [9]. In fact, FXI has a relatively minor role in hemostasis where it is primarily activated to FXIa by thrombin, and this process consolidates the final hemostatic clot through activation of FIX [9]. The classic pathways of blood coagulation including intrinsic, extrinsic, common pathways and the role of FXI are illustrated in Fig. 2.
Intrinsic Coagulation Pathway. The intrinsic system of coagulation, also referred to as the intrinsic pathway includes surface contact activating factors XII and XI. Activated factor XI (XIa) in the presence of calcium enzymatically converts factor IX to its activated form, IXa. When combined with Factor VIIIa, calcium, and phospholipid from vascular/cellular surfaces the tenase complex converts prothrombin to thrombin that in turn converts fibrinogen to fibrin. Fibrin is the primary component of hemostatic and pathological thrombosis. The extrinsic pathway of coagulation follows vascular injury and exposure of tissue factor (TF) that convert FVII to VIIa, converting FX to FXa (common pathway of coagulation), and generating thrombin for fibrin clot formation
Role of FXI in thrombosis
Unlike its more modest role in hemostasis, FXI plays a major role in pathological thrombus formation [10]. Pathological thrombosis can be triggered by two main pathways: the tissue factor pathway and the contact pathway. In either case, thrombin is generated during the initial phase, which subsequently activates Factor XI. In turn, FXI drives thrombus growth and propagation in a process called amplification [11]. This fundamental role of FXI in thrombosis is supported by several animal studies in which FXI-deficient or inhibited animal models had reduced incidence of thrombosis [12]. Furthermore, individuals with high levels of FXI were found to have a higher risk for venous thromboembolism (VTE) [13] while those with FXI deficiency had a lower incidence of DVT when compared to the general population [14].
Based on the different contributions of FXI in hemostasis and thrombosis discussed above, inhibition of FXI has emerged as a promising approach to uncouple the therapeutic benefits and the adverse effects of anticoagulant treatments; in other terms, by targeting FXI, it is possible that we might be able to reduce the risk of thrombotic complications without simultaneously increasing the likelihood of bleeding, which is a major concern with other types of anticoagulants.
General properties
Different inhibition strategies aimed at FXIa have been developed, and include antisense oligonucleotides, monoclonal antibodies, and small molecule inhibitors [15] (Fig. 3). Several of these inhibitors have demonstrated strong specificity for FXIa, leading to prolonged plasma clotting time, and have shown protective effects against thrombosis in different animal models without affecting the bleeding time [10, 16, 17]. The mechanisms, pharmacodynamics, and pharmacokinetics of FXI inhibitors that are tested in clinical trials are summarized in Table 1 [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. FXI inhibitors in different stages of development are illustrated in (Fig. 4).
Sites of Factor XI Inhibition for Drugs in Development. Some inhibitors target factor XI while others bind factor XI in its activated form (XIa). Anti-sense oligonucleotides (ASO) inhibit the hepatic synthesis of factor XI in the intracellular space. Factor (F), Ixodes ricinus contact-phase inhibitor (Ir-CPI). The classes of inhibitors include monoclonal antibodies, small molecules, aptamers, anti-sense oligonucleotides, and natural inhibitors
Antisense oligonucleotides
These drugs inhibit the synthesis of factor XI by binding to specific mRNA [34]. They are highly bound to plasma proteins with limited renal clearance. Additionally, they have a slow onset of action and long half-life. Therefore, and coupled with the 52-h half-life of FXI reversal of their action would require long-term FXI replacement with a factor XI concentrate [34]. IONIS-FXIRx was the first ASO drug that was tested in clinical trials [27, 35]. Another drug, fesomersen (LICA), a modified form of IONIS-FXIRx, is currently being studied [36].
Small molecules
These drugs are synthetic compounds with low molecular weight. They inhibit FXIa through binding to target proteins; hence, their effects cannot be countered by replacement products, as they do not reduce factor XI levels directly. In certain situations, activated prothrombin complex concentrate, recombinant factor VIIa, and antifibrinolytic agents might be considered as potential interventions to counteract the action of these drugs. They have a rapid onset and short duration of action and are amenable to renal clearance. Milvexian [9, 18, 37, 38] and asundexian [20, 24, 30,31,32, 39] are two main drugs that have been widely tested in human clinical trials. Others include SHR-2285 [11], frunexian [40], ONO-7648 [41], BMS-962212 [42], and Sulfated pentagalloylglucosides [43].
Monoclonal antibodies
This class of drug acts primarily by preventing activation of FXI to FXIa or by binding to FXIa and preventing its activity, however, there are other mechanisms as well. For example, Bay 1,831,865 hinders the binding of FXI to FIX, thereby preventing FXI activation. It also inhibits activation of FXI to FXIa [24]. Xisomab binds to FXI and to the A2 domain thereby preventing FXI activation by FXIIa [44]. The other functions of FXI are not impacted.
Monoclonal antibodies exhibit a rapid onset of action and a prolonged duration, with effects lasting over a month. Their metabolism relies mainly on the reticuloendothelial system [45]. In situations where their effects need to be reversed, the preferred method would be the use of tranexamic acid and agents that bypass FXI such as recombinant FVIIa (rFVIIa) or activated prothrombin complex concentrate (aPCC). Osocimab [46], Abelacimab [26], and Xisomab 3G3 [23] are three drugs that have been widely tested in human clinical trials. Other compounds that are under investigation or in the pre-clinical phase include BAY 1831865 [24, 47] and REGN9933 [48].
Natural peptides
Fasxiator [49] and Ir-CPI [50] are natural peptides isolated from snake Bungarus fasciatus and tick Ixodes Ricinus respectively. They inhibit the activation of FXI with a rapid onset of action and a short half-life. Other natural peptides like Desmolaris, acaNAP10, and Boophilin are currently in the initial study phases on a limited basis [51,52,53]. Natural peptides inhibitors have not been tested in humans to date.
Aptamers
DNA-based agents that inhibit either FXI or its activation to FXIa do so with considerable specificity, rapid onset of action and a short half-life [54]. They are in early stages of development and have not been tested in humans to date.
Published clinical trials on factor XI inhibitors
Several phase 2 clinical trials investigating the safety and efficacy of FXI inhibitors have been published. These trials focused on six clinical indications for the use of FXI inhibitors which were total knee arthroplasty (TKA), end-stage renal disease (ESRD), atrial fibrillation (AF), acute non-cardioembolic ischemic stroke, acute ischemic stroke, or high-risk transient ischemic attack (TIA) and acute myocardial infarction. A summary of the published phase 2 clinical trials including indication, intervention, control, population, efficacy, and safety outcomes is shown in (Table 2).
TKA
Four clinical trials involving four different drugs (IONIS-FXIRx, Osocimab, Abelacimab, and Milvexian) have been published [9, 25, 28, 35].
-
FXI-ASO TKA (NCT01713361): an open-label, parallel-group noninferiority randomized controlled trial in which 300 patients undergoing elective TKA, were randomly assigned to receive one of three doses of FXI-ASO (100 mg, 200 mg, and 300 mg) or 40 mg of enoxaparin once daily. The primary efficacy endpoint was the incidence of adjudicated total VTE, which was a composite of objectively confirmed symptomatic VTE, unexplained death for which pulmonary embolism could not be ruled out, and asymptomatic DVT (detected by mandatory bilateral venography at 8–12 days postoperatively). Major or clinically relevant nonmajor bleeding (CRNMB) were identified as the primary safety outcomes. Shortly after starting the trial, the 100-mg dose regimen was discontinued to ensure a sufficient reduction in factor XI levels during and after surgery. Treatment with FXI-ASO was initiated 36 days before surgery and patients received a total of 9 subcutaneous doses over a period of 39 days. Meanwhile, Enoxaparin, at a dose of 40 mg administered subcutaneously once daily, was initiated the evening before or 6 to 8 h after surgery and was to be continued for at least 8 days postoperatively. The incidence rates of VTE were quite comparable with 27% and 30% in 200 mg of FXI-ASO and enoxaparin groups, respectively, whereas the incidence was significantly reduced to only 4% in the 300 mg of FXI-ASO group. By contrast, the incidence of bleeding events was 3% in both groups of FXI-ASO compared to 8% in the enoxaparin group. Frequent occurrences of mild injection site reactions were noted among participants using FXI-ASO, but these reactions did not lead to the discontinuation of the study drug. The dose regimen's safety profile was further supported by the similarity of postoperative hemoglobin levels between FXI-ASO and enoxaparin users.
-
FOXTROT (NCT03276143): Randomized, open-label, adjudicator-blinded, phase 2 noninferiority trial in which 813 adult patients, who were undergoing unilateral knee arthroplasty, were randomly assigned to receive single intravenous osocimab postoperative doses of 0.3, 0.6, 1.2, or 1.8 mg/kg; preoperative doses of 0.3 or 1.8 mg/kg; or 40 mg of subcutaneous enoxaparin once daily or 2.5 mg of oral apixaban twice daily for at least 10 days or until venography. The primary outcome was VTE incidence (detected by mandatory bilateral venography at 10 to 13 days after surgery, confirmed symptomatic DVT, or pulmonary embolism). A noninferiority margin of 5% was selected for comparison with enoxaparin. The main safety outcome evaluated was the occurrence of major or CRNMB within 10 to 13 days after the surgery. The primary outcome occurred in 23.7% receiving 0.3 mg/kg, 15.7% receiving 0.6 mg/kg, 16.5% receiving 1.2 mg/kg, and 17.9% receiving 1.8 mg/kg of osocimab postoperatively; 29.9% receiving 0.3 mg/kg and 11.3% receiving 1.8 mg/kg of osocimab preoperatively; 26.3% receiving enoxaparin; and 14.5% receiving apixaban. Hence, postoperative osocimab 0.6 mg/kg, 1.2 mg/kg, and 1.8 mg/kg were deemed noninferior compared to enoxaparin, and the preoperative 1.8-mg/kg dose of osocimab showed superiority over enoxaparin; however, Postoperative and preoperative doses of 0.3 mg/kg of osocimab did not meet the predetermined criteria for noninferiority. The incidence of major or CRNMB was observed in up to 4.7% of patients receiving osocimab, 5.9% receiving enoxaparin, and 2% receiving apixaban. Bleeding was linked to the surgical site. No intracranial bleeding or bleeding into another critical site was observed.
-
ANT-005 TKA (EudraCT 2019–003756-37): an open-label, parallel-group noninferiority trial in which 412 patients who were undergoing TKA were randomly assigned to receive either a single intravenous dose of abelacimab (30 mg, 75 mg, or 150 mg) postoperatively or 40 mg of enoxaparin subcutaneously once daily. The primary efficacy outcome was VTE (detected by mandatory bilateral venography at 8 to 12 days after surgery, confirmed symptomatic DVT, or pulmonary embolism), and the primary safety outcome was major or CRNMB up to 30 days after surgery. Incidence of VTE was 13%, 5%, 4%, and 22% for 30, 75, 150 mg of abelacimab, and 40 mg of enoxaparin, respectively. Bleeding occurred in 2%, 2%, and 0% of the patients in the 30, 75, and 150 mg abelacimab groups, respectively, and no bleeding event was reported in the enoxaparin group. Serious adverse events occurred in 1%, 3%, and 1% of the patients in the 30, 75, and 150 mg abelacimab groups, respectively, and in none of the patients in the enoxaparin group. No anti-drug antibodies or hypersensitivity reactions were detected.
-
AXIOMATIC-TKR (NCT03891524): a parallel-group, phase 2 trial in which 1242 patients, who were undergoing knee arthroplasty, were randomly assigned to receive one of seven postoperative regimens of milvexian (25, 50, 100, or 200 mg twice daily or 25, 50, or 200 mg once daily) or enoxaparin (40 mg once daily). The primary efficacy outcome was VTE. The primary safety outcome was bleeding events whereas the primary efficacy endpoint was VTE incidence, which was a composite of asymptomatic DVT (detected by mandatory unilateral venography performed at 10 to 14 days postoperatively), confirmed symptomatic DVT, or death from any cause. The proof of efficacy was determined as a significant dose–response relationship and a venous thromboembolism incidence that was significantly lower than 30% with the twice-daily milvexian regimen. In the milvexian twice-daily group, venous thromboembolism developed in 21%, 11%, 9%, and 8% among patients received 25, 50, 100, and 200 mg respectively. On the other hand, in the milvexian once daily group, venous thromboembolism developed in 25%, 24%, and 7% among patients received 25, 50, and 200 mg respectively, as compared to 21% in patients taking enoxaparin. Bleeding of any severity was reported in 4% of patients taking milvexian; the same incidence with enoxaparin. Major or clinically relevant nonmajor bleeding was reported in 1% and 2%, respectively; and serious adverse events were reported in 2% and 4%, respectively. Milvexian increased the aPTT ratio in a dose-dependent manner, and no evidence of a dose-dependent increase in bleeding was noted.
It’s worth mentioning that the trials in patients undergoing TKA predominantly identified VTE events through screening venography 8-14 days after surgery, mostly asymptomatic and non-clinically significant which is an important limitation in these phase 2 clinical trials. Therefore, emphasis is placed on ongoing clinical trials targeting clinical endpoints in this patient group.
ESRD
Two clinical trials involving two different drugs IONIS-FXIRx) and Xisomab 3G3) have been published do date [23, 55].
-
Walsh et al. (NCT02553889): a phase 2b study enrolled 49 patients receiving hemodialysis. The study was composed of two parts. First, 6 individuals received 1 dose of IONIS-FXIRx 300 mg pre- and post-hemodialysis. Subsequently, 43 participants were allocated in a double-blind, randomized design to either 200 mg, 300 mg of IONIS-FXIRx, or placebo for 12 weeks. The principal goal was to evaluate the pharmacokinetics, pharmacodynamics, and adverse events. The PK of the drug was consistent with previous studies and similar whether injected pre- or post-hemodialysis. No accumulation of IONIS-FXIRx was observed after repeated administration. After 12 weeks, mean levels of FXI activity decreased by 56.0% and 70.7% in the 200 mg and 300 mg groups, respectively compared to a 3.9% reduction in the placebo group. Injection site reaction was the most common adverse event. Clinically relevant bleeding occurred in 0 (0.0%; 200 mg), 1 (6.7%; 300 mg), and 1 (7.7%; placebo) patients, respectively.
-
(RxFXI-formerly IONIS) The phase 2b study of fesomersen (formerly IONIS-FXIRx)achieved its primary outcome measure of no increase in incidence of major bleeding and clinically relevant non-major bleeding as compared to placebo. Data from the study show that fesomersen, administered monthly at 40 mg, 80 mg, and 120 mg for up to 48 weeks, was safe and well-tolerated. Fesomersen also demonstrated substantial and statistically significant reductions in Factor XI activity levels.
-
Lorentz et al. (NCT03612856): a randomized, double-blinded, phase 2 trial comparing Xisomab (AB023) to placebo in 24 patients with ESRD undergoing heparin-free hemodialysis. Patients were randomly allocated to receive a single dose of AB023 (0.25 or 0.5 mg/kg) or placebo before dialysis. The primary aim was to evaluate the safety of the drug about bleeding events. No clinically relevant bleeding event was reported in the study group, and the time to hemostasis at vascular access sites remains the same after drug administration. Furthermore, after the drug administration, there were reductions in levels of thrombin-antithrombin complexes and C-reactive protein, and there were fewer occlusive events that necessitated hemodialysis circuit exchange. So, AB023 effectively inhibited contact activation-induced coagulation, was well-tolerated, and resulted in decreased clotting within the dialyzer.
AF
One clinical trial was published so far investigating the use of asundexian in patients with AF [30].
-
PACIFIC-AF (NCT04218266): a randomized, double-blind, phase 2 study in which asundexian 20 mg or 50 mg once daily compared to apixaban 5 mg twice daily in 755 patients aged 45 years or older with atrial fibrillation, a CHA2DS2-VASc score of at least 2 if male or at least 3 if female (moderate to high risk for stroke), and increased bleeding risk. Patients were followed-up for 12 weeks. The primary endpoint was the composite of major or clinically relevant non-major bleeding according to the International Society on Thrombosis and Hemostasis (ISTH). The incidence of bleeding events was 1.2% and 0.4% in the asundexian 20 mg and 50 mg respectively compared to 2.4% in the apixaban group which represented a significant reduction in bleeding rates with the 50 mg asundexian regimen. On the other hand, asundexian at a 20 mg dose exhibited 81% inhibition of FXIa activity during trough concentrations and 90% inhibition during peak concentrations, whereas the 50 mg dose showed 92% inhibition during trough concentrations and 94% inhibition during peak concentrations. Regarding the exploratory thrombotic composite endpoint of cardiovascular death, myocardial infarction, ischemic stroke, or systemic embolism, a total of 2, 4, and 3 events occurred in asundexian 20 mg, asundexian 50 mg, and apixaban groups respectively. The small number of events in phase 2 did not raise an alarm, however, the IDMC recommended stopping the OCEANIC AF phase 3 trial due to inferior efficacy on November 19, 2023. The other trials were not impacted and are ongoing (bayer.com; accessed November 20, 2023).
Acute non-cardioembolic ischemic stroke
One clinical trial was published so far investigating the use of asundexian in patients with acute non-cardioembolic ischemic stroke [31].
-
PACIFIC-Stroke (NCT04304508): a randomized, double-blind, phase 2b study in which 1808 patients aged 45 years or older with non-cardioembolic ischemic stroke were randomly assigned to receive asundexian 10, 20, 50 mg once daily dose or placebo in addition to usual antiplatelet therapy. Patients were treated and followed up for 26-52 weeks. The composite of recurrent symptomatic ischemic stroke and MRI-detected brain infarcts after randomization was defined as a primary efficacy outcome. The primary safety outcome was major or CRNMB according to ISTH criteria. The incidence of bleeding events was observed in 4%, 3%, and 4% in the asundexian 10, 20, and 50 mg respectively compared to 2% in the placebo group. On the other hand, the primary efficacy outcome was observed in 19%, 22%, and 20% in the asundexian 10, 20, and 50 mg respectively compared to 19% in the placebo group. There was no dose-response association and no significant increase in the incidence of the primary safety outcome.
Acute ischemic stroke or high-risk TIA
One clinical trial was published so far investigating the use of milvexian in patients with acute ischemic stroke or high-risk TIA [38] (The results were presented at the European Society of Cardiology 2022 Conference; August 28, 2022).
-
AXIOMATIC-SSP (NCT03766581): a phase 2, randomized, double-blind, placebo-controlled trial examining the efficacy and safety of milvexian in 2366 patients with acute ischemic stroke or at high risk of TIA. The main objective was to estimate the dose-response relationship of milvexian on stroke occurrence and bleeding. Patients were randomly assigned to receive one of five doses of milvexian (25, 50, 100, 200 mg twice daily, or 25 mg once daily) or placebo daily for 90 days. All study participants received background treatment with aspirin and clopidogrel for 21 days, followed by aspirin from day 22 to 90. The primary efficacy outcome was the composite of ischemic stroke or incident infarct on brain MRI. The primary safety outcome was the major bleeding events defined as Bleeding Academic Research Consortium (BARC) type 3 or 5 bleeding. The primary efficacy outcome was lower at the 50 and 100 mg twice daily groups; however, there was no apparent dose-response relationship (placebo 16.6%, 25 mg once daily 16.2%, 25 mg twice daily 18.5%, 50 mg twice daily 14.1%, 100 mg twice daily 14.7%, 200 mg twice daily 16.4%). Additionally, milvexian numerically reduced the risk of clinical ischemic stroke in the intention-to-treat population at all doses except 200 mg twice daily, with doses from 25 to 100 mg twice daily showing an approximately 30% relative risk reduction compared to placebo (placebo 5.5%, 25 mg once daily 4.6%, 25 mg twice daily 3.8%, 50 mg twice daily 4.0%, 100 mg twice daily 3.5%, 200 mg twice daily 7.7%). On the other hand, the incidence of major bleeding was low overall (placebo 0.6%, 25 mg once daily 0.6%, 25 mg twice daily 0.6%, 50 mg twice daily 1.5%, 100 mg twice daily 1.6%, 200 mg twice daily 1.5%). The incidence of major bleeding for the milvexian 25 mg once and twice daily groups was like placebo, while a moderate increase in the 50 mg twice daily group and above with no dose-response relationship. There was no increase in severe bleeding (BARC type 3c or symptomatic intracranial hemorrhage) compared to placebo, and there was no fatal bleeding in any arm of the study.
Acute myocardial infarction
One clinical trial was published so far investigating the use of asundexian in patients with acute myocardial infarction [32].
-
PACIFIC-AMI (NCT04304534): a double-blind, placebo-controlled, phase 2, dose-ranging trial investigating the efficacy and safety of asundexian in 1601 patients with recent acute myocardial infarction. The primary efficacy outcome was a composite of cardiovascular death, MI, stroke, or stent thrombosis. The main safety outcome was BARC type 2, 3, or 5 bleeding. Patients were randomly assigned to receive oral asundexian 10, 20, or 50 mg or placebo once daily for 6 to 12 months. asundexian was associated with a dose-dependent reduction of FXIa activity with 50 mg of asundexian resulted in > 90% inhibition of baseline FXIa activity. There was no significant difference in the incidence of bleeding among different groups with incidence of 7.6%, 8.1%, 10.5%, and 9.0% in patients receiving asundexian 10 mg, 20 mg, 50 mg, and placebo respectively (pooled asundexian versus placebo: hazard ratio, 0.98 [90% CI, 0.71-1.35]). Whereas the efficacy outcome identified in 6.8%, 6.0%, 5.5%, and 5.5% in patients receiving asundexian 10 mg, 20 mg, 50 mg, and placebo respectively (pooled asundexian versus placebo: hazard ratio, 1.05 [90% CI, 0.69-1.63). Therefore, asundexian was associated with a dose-dependent, near-complete inhibition of FXIa activity without a significant increase in bleeding and a low rate of ischemic events.
Ongoing randomized controlled trials
Several randomized controlled trials are currently underway investigating FXI inhibitors’ efficacy and safety for VTE prophylaxis in different clinical conditions. These conditions include ESRD, ischemic stroke, AF, and cancer. A summary of these trials including drug name, control, number of participants, clinical scenario, outcome measures, follow-up period, and trials timelines is shown in (Table 3).
Conclusion
Despite the availability of various oral and parenteral anticoagulants for preventing venous and arterial thromboembolism, bleeding complications continue to pose challenges for both healthcare providers and patients. A novel approach in anticoagulation involves targeting the contact pathway at the level of FXI to uncouple hemostasis and thrombosis has emerged as a potential safer option. Promising results from the completed phase 2 studies of FXI inhibitors, including antisense oligonucleotides, monoclonal antibodies, and small molecules, indicate dose-dependent reductions in FXI concentrations and thrombotic complications without a corresponding increase in the bleeding risk. The ongoing phase 2 and 3 trials focusing on clinical endpoints will be crucial in defining the role of FXI inhibitors in the future and clearly delineating both their overall efficacy and safety profiles. These developments may potentially pave the way for safer and more effective anticoagulant therapies for many patients in the future. Moreover, the development of reversal agents especially for drugs with long half-lives is another important issue that should be investigated.
Future directions
The biological and pathophysiological foundation for FXI inhibitors and their development for clinical use is very strong. The initial focus for clinical indications is well-founded based on opportunities and unmet need-secondary prevention of ischemic stroke, atrial fibrillation, cancer-associated thrombosis, and both primary and secondary prevention in patients with ESRD. Each may be met with enthusiasm by researchers, clinicians, and patients alike. The ability of FXI inhibitors to replace DOACs in atrial fibrillation for example will require at the very least similar efficacy and superior safety in patients at moderate (or high) risk for both cardioembolic stroke and major bleeding. The use of FXI inhibitors in patients with ESRD, and hopefully those with moderately reduced kidney performance in the future, is particularly exciting given the high risk for thrombotic events at several anatomic sites and concomitant bleeding risk in these patients.
Data availability
The authors confirm that the data supporting the findings of these studies previously published are available within the article [and/or] its supplementary materials.
References
Bouma BN, Griffin JH (1977) Human blood coagulation factor XI. Purification, properties, and mechanism of activation by activated factor XII. J Biol Chem 252:6432–6437
Ho DH, Badellino K, Baglia FA, Sun M-F, Zhao M-M, Gailani D, Walsh PN (2000) The role of high molecular weight kininogen and prothrombin as cofactors in the binding of factor XI A3 domain to the platelet surface. J Biol Chem 275:25139–25145
Sun Y, Gailani D (1996) Identification of a factor IX binding site on the third apple domain of activated factor XI. J Biol Chem 271:29023–29028
Baglia FA, Walsh PN (2007) Prothrombin is a cofactor for the binding of factor XI to the platelet surface and for platelet-mediated factor-XI activation by thrombin. Biochemistry 46:12886–12887
Papagrigoriou E, McEwan PA, Walsh PN, Emsley J (2006) Crystal structure of the factor XI zymogen reveals a pathway for transactivation. Nat Struct Mol Biol 13:557–558
McMullen BA, Fujikawa K, Davie EW (1991) Location of the disulfide bonds in human coagulation factor XI: the presence of tandem apple domains. Biochemistry 30:2056–2060. https://doi.org/10.1021/bi00222a008
Wang X, Smith P, Hsu MY, Gailani D, Schumacher W, Ogletree M, Seiffert D (2006) Effects of factor XI deficiency on ferric chloride-induced vena cava thrombosis in mice. J Thromb Haemost 4:1982–1988
Wu W, Sinha D, Shikov S, Yip CK, Walz T, Billings PC, Lear JD, Walsh PN (2008) Factor XI homodimer structure is essential for normal proteolytic activation by factor XIIa, thrombin, and factor XIa. J Biol Chem 283:18655–18664
Hsu C, Hutt E, Bloomfield DM, Gailani D, Weitz JI (2021) Factor XI inhibition to uncouple thrombosis from hemostasis: JACC review topic of the week. J Am Coll Cardiol 78:625–631
Woodruff RS, Sullenger B, Becker RC (2011) The many faces of the contact pathway and their role in thrombosis. J Thromb Thrombolysis 32:9–20. https://doi.org/10.1007/s11239-011-0578-5
Greco A, Laudani C, Spagnolo M, Agnello F, Faro DC, Finocchiaro S, Legnazzi M, Mauro MS, Mazzone PM, Occhipinti G (2023) Pharmacology and clinical development of factor XI inhibitors. Circulation 147:897–913
Yau JW, Liao P, Fredenburgh JC, Stafford AR, Revenko AS, Monia BP, Weitz JI (2014) Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood 123:2102–2107
Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR (2000) High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med 342:696–701
Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U (2011) Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost 105:269–273
Schumacher WA, Luettgen JM, Quan ML, Seiffert DA (2010) Inhibition of factor XIa as a new approach to anticoagulation. Arterioscler Thromb Vasc Biol 30:388–392
Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer H-U, Burfeind P, Renné C, Gailani D, Nieswandt B, Renné T (2006) Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med 203:513–518
Takahashi M, Yamashita A, Moriguchi-Goto S, Sugita C, Matsumoto T, Matsuda S, Sato Y, Kitazawa T, Hattori K, Shima M (2010) Inhibition of factor XI reduces thrombus formation in rabbit jugular vein under endothelial denudation and/or blood stasis. Thromb Res 125:464–470
Perera V, Wang Z, Luettgen J, Li D, DeSouza M, Cerra M, Seiffert D (2022) First-in-human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci 15:330–342
Thomas D, Kanefendt F, Schwers S, Unger S, Yassen A, Boxnick S (2021) First evaluation of the safety, pharmacokinetics, and pharmacodynamics of BAY 2433334, a small molecule targeting coagulation factor XIa. J Thromb Haemost 19:2407–2416
Kubitza D, Heckmann M, Distler J, Koechel A, Schwers S, Kanefendt F (2022) Pharmacokinetics, pharmacodynamics and safety of BAY 2433334, a novel activated factor XI inhibitor, in healthy volunteers: a randomized phase 1 multiple-dose study. Br J Clin Pharmacol 88:3447–3462
Büller HR, Gailani D, Weitz JI (2015) Factor XI antisense oligonucleotide for venous thrombosis. N Engl J Med 372:1672–1672
Chan NC, Weitz JI (2019) AB023, a novel antibody that binds factor XI and blocks its activation by factor XIIa. Arterioscler Thromb Vasc Biol 39:533–535
Lorentz CU, Verbout NG, Wallisch M, Hagen MW, Shatzel JJ, Olson SR, Puy C, Hinds MT, McCarty OJ, Gailani D (2019) Contact activation inhibitor and factor XI antibody, AB023, produces safe, dose-dependent anticoagulation in a phase 1 first-in-human trial. Arterioscler Thromb Vasc Biol 39:799–809
Nowotny B, Thomas D, Schwers S, Wiegmann S, Prange W, Yassen A, Boxnick S (2022) First randomized evaluation of safety, pharmacodynamics, and pharmacokinetics of BAY 1831865, an antibody targeting coagulation factor XI and factor XIa, in healthy men. J Thromb Haemost 20:1684–1695. https://doi.org/10.1111/jth.15744
Weitz JI, Chan NC (2020) Novel antithrombotic strategies for treatment of venous thromboembolism. Blood 135:351–359
Yi BA, Freedholm D, Widener N, Wang X, Simard E, Cullen C, Al-Saady NM, Lepor NE, Coulter S, Lovern M (2022) Pharmacokinetics and pharmacodynamics of abelacimab (MAA868), a novel dual inhibitor of factor XI and factor XIa. J Thromb Haemost 20:307–315
Walsh M, Bethune C, Smyth A, Tyrwhitt J, Jung SW, Rosie ZY, Wang Y, Geary RS, Weitz J, Bhanot S (2022) Phase 2 study of the factor XI antisense inhibitor IONIS-FXIRx in patients with ESRD. Kidney Int Rep 7:200–209
Verhamme P, Yi BA, Segers A, Salter J, Bloomfield D, Büller HR, Raskob GE, Weitz JI (2021) Abelacimab for prevention of venous thromboembolism. N Engl J Med 385:609–617
Weitz JI, Eikelboom JW (2022) What is the future of factor XI inhibitors? Circulation 146:1899–1902
Piccini JP, Caso V, Connolly SJ, Fox KA, Oldgren J, Jones WS, Gorog DA, Durdil V, Viethen T, Neumann C (2022) Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 399:1383–1390
Shoamanesh A, Mundl H, Smith EE, Masjuan J, Milanov I, Hirano T, Agafina A, Campbell B, Caso V, Mas J-L (2022) Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet 400:997–1007
Rao SV, Kirsch B, Bhatt DL, Budaj A, Coppolecchia R, Eikelboom J, James SK, Jones WS, Merkely B, Keller L (2022) A multicenter, phase 2, randomized, placebo-controlled, double-blind, parallel-group, dose-finding trial of the oral factor XIa inhibitor asundexian to prevent adverse cardiovascular outcomes after acute myocardial infarction. Circulation 146:1196–1206
Sharma M, Molina CA, Toyoda K, Bereczki D, Kasner SE, Lutsep HL, Tsivgoulis G, Ntaios G, Czlonkowska A, Shuaib A (2022) Rationale and design of the AXIOMATIC-SSP phase II trial: antithrombotic treatment with factor XIa inhibition to optimize management of acute thromboembolic events for secondary stroke prevention. J Stroke Cerebrovasc Dis 31:106742
Zhang H, Löwenberg EC, Crosby JR, MacLeod AR, Zhao C, Gao D, Black C, Revenko AS, Meijers JC, Stroes ES (2010) Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood 116:4684–4692
Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI (2015) Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 372:232–240
Willmann S, Marostica E, Snelder N, Solms A, Jensen M, Lobmeyer M, Lensing AW, Bethune C, Morgan E, Yu RZ (2021) PK/PD modeling of FXI antisense oligonucleotides to bridge the dose‐FXI activity relation from healthy volunteers to end‐stage renal disease patients. CPT: Pharmacometrics Syst Pharmacol 10:890–901
Wong PC, Crain EJ, Bozarth JM, Wu Y, Dilger AK, Wexler RR, Ewing WR, Gordon D, Luettgen JM (2022) Milvexian, an orally bioavailable, small-molecule, reversible, direct inhibitor of factor XIa: in vitro studies and in vivo evaluation in experimental thrombosis in rabbits. J Thromb Haemost 20:399–408
Sharma M, Molina CA, Toyoda K, Bereczki D, Bangdiwala SI, Kasner SE, Lutsep HL, Tsivgoulis G, Ntaios G, Czlonkowska A et al (2024) Safety and efficacy of factor XIa inhibition with milvexian for secondary stroke prevention (AXIOMATIC-SSP): a phase 2, international, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol 23:46–59. https://doi.org/10.1016/S1474-4422(23)00403-9
Heitmeier S, Visser M, Tersteegen A, Dietze-Torres J, Glunz J, Gerdes C, Laux V, Stampfuss J, Roehrig S (2022) Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa. J Thromb Haemost 20:1400–1411
Pollack CV Jr, Kurz MA, Hayward NJ (2020) EP-7041, a factor XIa inhibitor as a potential antithrombotic strategy in extracorporeal membrane oxygenation: a brief report. Crit Care Explor 2:e0196
Beale D, Dennison J, Boyce M, Mazzo F, Honda N, Smith P, Bruce M (2021) ONO-7684 a novel oral FXIa inhibitor: safety, tolerability, pharmacokinetics and pharmacodynamics in a first-in-human study. Br J Clin Pharmacol 87:3177–3189
Perera V, Luettgen JM, Wang Z, Frost CE, Yones C, Russo C, Lee J, Zhao Y, LaCreta FP, Ma X (2018) First-in-human study to assess the safety, pharmacokinetics and pharmacodynamics of BMS-962212, a direct, reversible, small molecule factor XIa inhibitor in non-Japanese and Japanese healthy subjects. Br J Clin Pharmacol 84:876–887
Al-Horani RA, Factor XI (2020) a) inhibitors for thrombosis: an updated patent review (2016-present). Expert Opin Ther Pat 30:39–55
Chan NC, Weitz JI (2019) AB023, a novel antibody that binds factor XI and blocks its activation by factor XIIa. Arterioscler Thromb Vasc Biol 39:533–535. https://doi.org/10.1161/ATVBAHA.119.312459
Tabrizi MA, Tseng C-ML, Roskos LK (2006) Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 11:81–88
Beavers CJ, Wayne NB (2020) Osocimab: a novel agent in preventing venous thromboembolism. J Cardiovasc Pharmacol 76:645–649
Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR (2009) Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood 113:936–944
Xie Z, Meng Z, Yang X, Duan Y, Wang Q, Liao C (2023) Factor XIa inhibitors in anticoagulation therapy: recent advances and perspectives. J Med Chem 66:5332–5363
Chen W, Carvalho L, Chan M, Kini R, Kang T (2015) Fasxiator, a novel factor XIa inhibitor from snake venom, and its site-specific mutagenesis to improve potency and selectivity. J Thromb Haemost 13:248–261
Decrem Y, Rath G, Blasioli V, Cauchie P, Robert S, Beaufays J, Frère J-M, Feron O, Dogné J-M, Dessy C (2009) Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med 206:2381–2395
Ma D, Mizurini DM, Assumpção TC, Li Y, Qi Y, Kotsyfakis M, Ribeiro JM, Monteiro RQ, Francischetti IM (2013) Desmolaris, a novel factor XIa anticoagulant from the salivary gland of the vampire bat (Desmodus rotundus) inhibits inflammation and thrombosis in vivo. Blood 122:4094–4106
Li D, He Q, Kang T, Yin H, Jin X, Li H, Gan W, Yang C, Hu J, Wu Y (2010) Identification of an anticoagulant peptide that inhibits both fXIa and fVIIa/tissue factor from the blood-feeding nematode Ancylostoma caninum. Biochem Biophys Res Commun 392:155–159
Assumpcao TC, Ma D, Mizurini DM, Kini RM, Ribeiro JM, Kotsyfakis M, Monteiro RQ, Francischetti IM (2016) In vitro mode of action and anti-thrombotic activity of boophilin, a multifunctional Kunitz protease inhibitor from the midgut of a tick vector of babesiosis, Rhipicephalus microplus. PLoS Negl Trop Dis 10:e0004298
Donkor DA, Bhakta V, Eltringham-Smith LJ, Stafford AR, Weitz JI, Sheffield WP (2017) Selection and characterization of a DNA aptamer inhibiting coagulation factor XIa. Sci Rep 7:2102
Walsh PN, Sinha D, Koshy A, Seaman FS, Bradford H (1986) Functional characterization of platelet-bound factor XIa: retention of factor XIa activity on the platelet surface. Blood 68:225–230
Buller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI (2015) Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 372:232–240. https://doi.org/10.1056/NEJMoa1405760
Weitz JI, Strony J, Ageno W, Gailani D, Hylek EM, Lassen MR, Mahaffey KW, Notani RS, Roberts R, Segers A (2021) Milvexian for the prevention of venous thromboembolism. N Engl J Med 385:2161–2172
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, A.E., Becker, R.C. Factor XI: structure, function and therapeutic inhibition. J Thromb Thrombolysis (2024). https://doi.org/10.1007/s11239-024-02972-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s11239-024-02972-5