Abstract
Introduction
There are a paucity of real-world data examining effectiveness and safety of non-vitamin K antagonist oral anticoagulants (NOACs) and warfarin in nonvalvular atrial fibrillation (NVAF) patients with prior bleeding.
Methods
This retrospective analysis included data from 5 insurance claims databases and included NVAF patients prescribed OACs with prior bleeding. One-to-one propensity score matching was conducted between NOACs and warfarin and between NOACs in each database. Cox proportional hazards models were used to evaluate the risk of stroke/systemic embolism (SE) and MB.
Results
A total of 244,563 patients (mean age 77; 50% female) with prior bleeding included 55,094 (22.5%) treated with apixaban, 12,500 (5.1%) with dabigatran, 38,246 (15.6%) with rivaroxaban, and 138,723 (56.7%) with warfarin. Apixaban (hazard ratio [HR]: 0.76 [95% CI: 0.70, 0.83]) and rivaroxaban (HR: 0.79 [95% CI: 0.71, 0.87]) had a lower risk of stroke/SE vs. warfarin. Apixaban (HR: 0.67 [95% CI: 0.64, 0.70]) and dabigatran (HR: 0.88 [95% CI: 0.81, 0.96]) had a lower risk of MB vs. warfarin. Apixaban patients had a lower risk of stroke/SE vs. dabigatran (HR: 0.70 [95% CI: 0.57, 0.86]) and rivaroxaban (HR: 0.85 [95% CI: 0.76, 0.96]) and a lower risk of MB than dabigatran (HR: 0.73 [95% CI: 0.67, 0.81]) and rivaroxaban (HR: 0.64 [95% CI: 0.61, 0.68]).
Conclusions
In this real-world analysis of a large sample of NVAF patients with prior bleeding, NOACs were associated with similar or lower risk of stroke/SE and MB vs. warfarin and variable risk of stroke/SE and MB against each other.
Highlights
-
Data on NOAC effectiveness and safety in NVAF patients with prior bleed history are lacking.
-
This study included data on OAC-treated NVAF patients with a history of bleeding.
-
NOACs were associated with similar or lower risk of stroke/SE and MB vs. warfarin.
-
NOACs were associated with variable risk of stroke/SE and MB against each other.
-
This study further demonstrated the effectiveness and safety profile when comparing NOACs to warfarin. The findings could aid to inform the discussion on the benefits and risks in the decision making process for NVAF patients who had a prior bleed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most commonly treated cardiac arrhythmia globally, with a major impact on healthcare costs.[1] The high risk of stroke and mortality following AF diagnosis is concerning. In emergency department settings, about 4% of patients experience stroke within one year of AF diagnosis, and about 11% die within that same time frame (8% due to stroke).[2] The complexity of AF needs a holistic approach with multidisciplinary, integrated management with active involvement of AF patients.[3] This integrated approach to patient evaluation and management is increasingly advocated for AF patients[4] given the beneficial impact on clinical outcomes.[5, 6].
A history of bleeding in the context of AF presents challenges for clinical management. AF patients with prior serious hemorrhagic events, like gastrointestinal (GI) bleed or intracranial hemorrhage (ICH), are at an increased risk for subsequent serious hemorrhagic events.[7, 8] Resumption of anticoagulation therapy in AF patients following a major bleeding (MB) event may lower the risk of ischemic events and all-cause mortality[7, 9,10,11,12,13,14]; however, studies have found a high risk of recurrent MB when resuming oral anticoagulants (OAC), [13,14,15,16] thus presenting a clinical challenge. The clinician must therefore weigh the antithrombotic benefits of anticoagulation therapy against the possibility of incurring another major hemorrhagic event should therapy resume.
Vitamin K antagonists (VKAs), such as warfarin used to be the standard of care for stroke prevention in patients with non-valvular AF (NVAF).[17] The advent of the non-vitamin K antagonist OACs (NOACs) apixaban, dabigatran, edoxaban, and rivaroxaban has provided a convenient, efficacious, and tolerable alternative to anticoagulation with warfarin.[18] Unsurprisingly, the NOACs are increasingly used in everyday clinical practice.[19, 20].
Because of these differences, it is essential to evaluate whether AF patients with a history of bleeding might have different outcomes when they are treated with NOACs vs. warfarin. Additionally, as the uptake of NOACs continues to increase, more data will be needed to fully understand the risk–benefit profiles associated with each NOAC.
To date, research about anticoagulant therapy in AF following a major hemorrhagic event has generally focused on warfarin therapy alone or warfarin vs. NOACs collectively rather than comparing the individual NOACs to warfarin or to one another.[7, 12, 21,22,23] This is a critical omission, as pharmacokinetic differences among NOACs may affect their respective efficacy and safety. Further, given that the effectiveness and tolerability of pharmacotherapy in patients with NVAF can be influenced by pre-existing patient comorbidities, such as a history of bleeding, information on this specific subset of the AF population could be significant when making therapeutic decisions. To help address these gaps, this study assessed stroke/SE and MB associated with NOACs vs. warfarin and vs. one another among NVAF patients with prior bleeding.
Methods
Data sources
This was a retrospective observational data analysis of NVAF patients with a history of bleeding who received treatment with NOACs (i.e., apixaban, dabigatran, edoxaban, or rivaroxaban) or warfarin. Data were pooled from a sample of more than 180 million beneficiaries (~ 56% of the US population) using the five largest insurance databases in the US: Fee-for-Service Medicare data from the U.S. Centers for Medicare & Medicaid Services (CMS), the IBM Watson Health MarketScan Commercial Claims and Encounter (“MarketScan”), the IQVIA PharMetrics Plus™ Database (“PharMetrics”), the Optum Clinformatics™ Data Mart (“Optum”), and the Humana Research Database (“Humana”). Patients with Medicare Fee-for-Service, Medicare Advantage, and commercial insurance were included. Database records included demographic and clinical information and International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9-CM and ICD-10-CM) codes, Healthcare Common Procedure Coding System (HCPCS) codes, and National Drug Codes.[24].
Patient selection
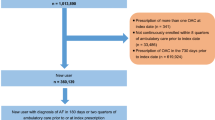
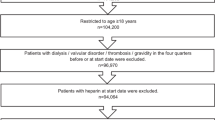
Adult patients (age ≥ 18 on index date) with an OAC treatment episode (apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin) between January 1, 2013 and June 30, 2019 (identification period) were selected. A treatment episode was defined as the treatment from OAC prescription date to discontinuation (> 30 days with no OAC use), switch, death, the end of study period, or end of continuous medical or pharmacy enrollment. Episodes were included if the patient had an AF diagnosis during the 12 months prior to / on the OAC prescription date and continuous medical and pharmacy health plan enrollment for 12 months before or on the OAC prescription date (baseline period). Episodes were excluded if the patients had evidence of valvular heart disease, venous thromboembolism, transient AF (pericarditis, hyperthyroidism, thyrotoxicity), or heart valve replacement during the baseline period; were pregnant during the study period; or underwent hip or knee replacement surgery within 6 weeks before the OAC prescription date. Additional patient selection criteria are provided in Fig. 1.
Among patients with eligible OAC episodes, those with a bleeding event prior to or during the OAC treatment episode were selected. A bleeding event was defined as a hospitalization with a diagnosis of ICH or GI bleeding or a hospitalization with a primary diagnosis of bleeding at other key sites (e.g., conjunctival, genitourinary system, hematuria. The full list of codes used for other key sites can be found in Supplemental Table 1). If a patient had more than one type of bleed during baseline, a hierarchy was applied to categorize patients as follows: ICH, GI, and bleeding at other key sites. The first OAC prescription date after a bleeding event was designated as the index date. If bleeding event occurs during an OAC episode, the index date reflects the first prescription after the bleeding event within a treatment episode. If the bleeding event occurs prior to OAC treatment episode, the index date reflects the start of an OAC treatment episode. Patients prescribed edoxaban were excluded due to small sample size.
Outcome measures
The primary effectiveness outcome was stroke/systemic embolism (SE), stratified by stroke type (i.e., ischemic, hemorrhagic, and SE). The primary safety outcome was MB, stratified by GI bleeding, ICH, and MB in other key sites.[25, 26] Primary outcomes were operationalized by inpatient claims with stroke/SE or MB as the principal (Medicare, MarketScan, and Optum) or first-listed (Humana and PharMetrics) diagnosis. Diagnosis codes for stroke/SE and MB are presented in Supplemental Table 1.
Outcomes were assessed during the follow-up period, defined as the time from 1 day post-index date to the earliest of the following: 30 days post-discontinuation date, switch date (date of new OAC within 30-days of end of days supply of index OAC), date of death (inpatient and all-cause death for commercial data and Medicare populations, respectively), end of continuous health plan enrollment, or study end (June 30, 2019).
Statistical analysis
Descriptive analysis was conducted for each treatment cohort. To control for different patient characteristics, propensity score matching (PSM) was used to compare each individual NOAC with warfarin (i.e., apixaban vs. warfarin, dabigatran vs. warfarin, and rivaroxaban vs. warfarin) as well as each individual NOAC with one another (i.e., apixaban vs. dabigatran, apixaban vs. rivaroxaban, and dabigatran vs. rivaroxaban). PSM was conducted in each database using two comparative cohorts before pooling the datasets. Patients were matched 1:1 by propensity scores generated using multivariable logistic regressions for baseline characteristics, including type of prior bleed, prior OAC use, demographics, and clinical characteristics (see Tables 1 and 2 for complete covariate list). Further details on PSM methodology appear in prior publications.[27] The PSM-adjusted baseline variables were compared based on standardized differences, with a threshold of 10%.[28].
Stroke/SE and MB incidence after index OAC were calculated using the number of events divided by total person-years at risk and multiplied by 100, with Kaplan-Meier curves to illustrate cumulative rates. Cox proportional hazard models with robust sandwich estimates were also applied to the PSM population within the pooled dataset to evaluate the comparative risks.[29] OAC treatment was included as the independent variable in the Cox models because all matched covariates were similar after PSM between the 2 comparative arms. P-values of 0.05 were used as the threshold for statistical significance.
Subgroup Analysis
Three subgroup analyses were conducted. The first two subgroup analyses were two interaction analyses, one between treatment and prior OAC use (with prior OAC use vs. without prior OAC use), and another between treatment and type of prior bleed (i.e., ICH, GI, other). The statistical significance (p < 0.10) of the interaction between treatment and prior OAC use or bleed type was evaluated.
The third analysis was the dose subgroup analysis for the NOAC cohorts. Standard-dose (i.e., apixaban 5 mg twice-daily, dabigatran 150 mg twice-daily, rivaroxaban 20 mg daily) and lower-dose (apixaban 2.5 mg twice-daily, dabigatran 75/110 mg twice-daily, rivaroxaban 15 mg/10 mg daily) patients were examined separately based on index prescription dosage. Warfarin cohort patients were matched to NOAC patients with either dosage. INR data was not available for this analysis. The statistical methods of the main analysis were used, wherein 1:1 PSM patients in each dataset were pooled and compared.
Institutional Review Board approval
Institutional Review Board review and approval were not required because this study did not involve the collection, use, or transmittal of individually identifiable data. Both the datasets and the security of the offices where analysis was completed (and where the datasets are kept) meet the requirements of the Health Insurance Portability and Accountability Act of 1996.
Results
After applying the selection criteria, a total of 244,563 NVAF patients with prior bleeding events were identified, including 55,094 (22.5%) prescribed apixaban, 12,500 (5.1%) dabigatran, 38,246 (15.6%) rivaroxaban, and 138,723 (56.7%) warfarinFootnote 1. Among patients with a prior bleed, 60.0% had a prior GI bleed, 12.2% had a prior ICH bleed, and 27.9% had a bleed at another key site. Most patients had the bleeding event more than or equal to one year before the index date (65.4%) and had OAC treatment in the 12 months before the bleeding event (67.7%). For apixaban, dabigatran, and rivaroxaban patients, 38.8%, 31.5%, and 42.3% used lower dosage regimens, respectively. The baseline characteristics of patients in each treatment cohort can be found in Supplemental Table 2.
The unadjusted incidence rate of stroke/SE—including ischemic stroke, hemorrhagic stroke, and SE—was 2.7 (apixaban), 2.6 (dabigatran), 2.5 (rivaroxaban), and 2.9 (warfarin) per 100 person-years (data not shown). The unadjusted incidence rate of MB—including GI bleeding, ICH, and other MB—was 9.4 (apixaban), 10.9 (dabigatran), 13.4 (rivaroxaban), and 13.6 (warfarin) per 100 person-years, respectively (data not shown).
After 1:1 PSM, a total of 50,435 apixaban–warfarin, 12,436 dabigatran–warfarin, 37,405 rivaroxaban–warfarin, 12,275 apixaban–dabigatran, 35,376 apixaban–rivaroxaban, and 12,297 dabigatran–rivaroxaban pairs were evaluated. The mean age was 77–78 years for the matched cohorts, and the mean follow-up time was 8–9 months. Complete descriptive baseline characteristics of the pooled analysis are presented in Tables 1 and 2. All baseline variables included in the PSM logistic models were balanced with standardized differences < 10% (Tables 1 and 2).
NOAC–Warfarin comparisons after PSM
Among NVAF patients with prior bleed, apixaban (hazard ratio [HR]: 0.76, 95% confidence interval [CI]: 0.70–0.83) and rivaroxaban use (HR: 0.79, 95% CI: 0.71–0.87) were associated with a lower risk of stroke/SE compared with warfarin. Ischemic stroke was the most prevalent type of stroke/SE, with a lower risk in apixaban (HR: 0.83, 95% CI: 0.75–0.91) and rivaroxaban (HR: 0.84, 95% CI: 0.75–0.94) patients compared with warfarin patients. (Fig. 2).
Propensity Score-Matched Incidence Rates and Hazard Ratios of Stroke/SE and Major Bleeding for NOAC versus Warfarin
Cox proportional hazard models with robust sandwich estimates were used to evaluate the risk of stroke/SE and major bleeding
CI: confidence interval; GI: gastrointestinal; ICH: intracranial hemorrhage; NOAC: non-vitamin K antagonist oral anticoagulant; SE: systemic embolism
Apixaban (HR: 0.67, 95% CI: 0.64–0.70) and dabigatran (HR: 0.88, 95% CI: 0.81–0.96) were associated with a lower risk of MB compared with warfarin. Apixaban was associated with a lower risk (HR:0.75, 95% CI: 0.71–0.79), and rivaroxaban was associated with a higher risk (HR: 1.17, 95% CI:1.10–1.25) of GI bleeding (the most prevalent type of MB) vs. warfarin. All NOACs were associated with a lower risk of ICH vs. warfarin (apixaban: HR: 0.67, 95% CI: 0.59–0.76; dabigatran: HR: 0.66, 95% CI: 0.51–0.86; rivaroxaban: HR: 0.64, 95% CI: 0.55–0.74). (Fig. 2).
NOAC–NOAC comparisons after PSM
Apixaban patients had a lower risk of stroke/SE compared with dabigatran (HR: 0.70, 95% CI: 0.57–0.86) and rivaroxaban (HR: 0.85, 95% CI: 0.76–0.96), and dabigatran patients were associated with a similar risk of stroke/SE compared with rivaroxaban (HR: 1.04, 95% CI: 0.87–1.25) (Fig. 3). Compared with dabigatran and rivaroxaban, apixaban was associated with a lower risk of MB (dabigatran: HR: 0.73, 95% CI: 0.67–0.81, rivaroxaban: HR: 0.64, 95% CI: 0.61–0.68) and lower risk of GI bleeding (dabigatran HR: 0.75, 95% CI: 0.67–0.85 and rivaroxaban HR: 0.64, 95% CI: 0.59–0.68). Compared with rivaroxaban, dabigatran was associated with a lower risk of MB and GI bleeding (MB HR: 0.84, 95% CI: 0.77–0.92 and GI HR: 0.81, 95% CI: 0.73–0.90) (Fig. 3).
Propensity Score-Matched Incidence Rates and Hazard Ratios of Stroke/SE and Major Bleeding for NOAC Comparisons
Cox proportional hazard models with robust sandwich estimates were used to evaluate the risk of stroke/SE and major bleeding
CI: confidence interval; GI: gastrointestinal; ICH: intracranial hemorrhage; NOAC: non-vitamin K antagonist oral anticoagulant; SE: systemic embolism
The Kaplan-Meier curves for cumulative incidence of stroke/SE and MB in the matched populations appear in Supplemental Figs. 1 and 2.
Subgroup analyses
In the first interaction analysis of treatment with prior OAC use, treatment effects were generally consistent regardless of prior OAC use. For dabigatran vs. warfarin and dabigatran vs. rivaroxaban, patients without prior OAC use experienced a greater magnitude of reduction in the risk of MB compared with patients with prior OAC use. Additionally, for apixaban vs. dabigatran, a similar risk of MB was observed among patients without prior OAC use while a lower risk of MB was found in those with prior OAC use (Supplemental Tables 3 and 4). No significant interactions were observed for treatment and type of prior bleed for stroke/SE or MB (Supplemental Tables 5 and 6).
Results of the dose subgroup analysis were generally consistent with the main analysis; however, the risk of stroke/SE was similar between standard-dose apixaban when compared with standard-dose rivaroxaban (Supplemental Table 7). Among patients with low-dose rivaroxaban, the risk of stroke/SE was similar compared with warfarin. There was no significant differences for stroke/SE between the low-dose NOACs [i.e. apixaban vs. rivaroxaban and apixaban vs. dabigatran] (Supplemental Table 8). Also, there was no significant difference in the risk of MB when comparing low dose dabigatran to rivaroxaban (Supplemental Table 8).
Discussion
To our knowledge, this is one of the first retrospective, real-world cohort analyses among U.S. patients to compare individual NOACs to warfarin and to one another in a large sample of NVAF patients with previous bleeding. Leveraging data from 5 large U.S. claims databases, this study found that apixaban and rivaroxaban were associated with a lower risk of stroke/SE, and dabigatran was associated with a similar risk of stroke/SE, when compared with warfarin. Apixaban and dabigatran were associated with a lower risk of MB, and rivaroxaban was associated with a similar risk of MB, compared with warfarin. Subgroup analyses of prior OAC use, type of prior bleed and NOAC dose showed generally consistent findings to the main analysis.
The current findings are consistent with published studies reporting favorable outcomes on stroke/SE and/or MB for NOACs vs. warfarin in AF patients with prior major hemorrhage.[22, 23, 30,31,32] Most of these studies used datasets from Danish, Korean, or Taiwanese populations, which may limit the generalizability of findings to U.S. patients. For example, Lee et al.[32] found NOACs were associated with multiple positive outcomes compared with warfarin in AF patients with previous ICH, including a lower risk of fatal and nonfatal ischemic stroke, ICH, the composite outcome of stroke plus ICH, death from the composite outcome, and all-cause mortality. Kwon et al.[31] similarly observed significantly lower rates of fatal and nonfatal ischemic stroke, fatal and nonfatal ICH, nonfatal GI bleed, and all-cause death with NOACs vs. warfarin in AF patients with a prior GI bleed.
The current study extends the findings from the existing evidence by comparing each NOAC individually against warfarin and against one another and using a large U.S. cohort that includes multiple types of bleeding (i.e., ICH, GI, and other MB). Our findings suggest NOACs may represent a safe and effective option for initiating or resuming anticoagulation in AF patients with prior bleeding, and that, compared with warfarin, these drugs could offer at least comparable— and in some cases possibly better—protection against stroke/SE and MB. However, these findings need to be confirmed by randomized controlled trials in AF patients with a history of ICH, GI bleed, or other MB. Some ongoing and recently completed randomized clinical trials will provide more insights about the effects of NOACs on thromboembolic and bleeding events in AF patients with a history of ICH (NCT03996772 and NCT02998905).
Across different NOACs, apixaban was associated with a lower risk of stroke/SE and MB compared with dabigatran and rivaroxaban, and dabigatran was associated with lower risk of MB than rivaroxaban. Our findings were consistent with Kwon et al.[31] who reported a lower risk of MB with apixaban vs. dabigatran, rivaroxaban, and edoxaban. Nonetheless, only head-to-head clinical trials will provide definitive answers about the efficacy and safety of NOACs vs. NOACs in the AF population, and in AF patients with a history of bleeding specifically.
The effectiveness and safety of different NOACs have not been previously established in a U.S. cohort of NVAF patients with prior bleeding. This represents a major literature gap, given that NOAC prescribing in the United States and Europe has increased considerably over the past decade,[33,34,35] with the American College of Cardiology, American Heart Association, and European Society of Cardiology now recommending NOACs over warfarin to reduce stroke risk in AF populations.[36,37,38] Formal clinical practice guidelines are still lacking as to which NOAC to prescribe, when, and at what dose for AF patients with prior bleeding. In response to growing evidence about the benefits of NOACs, cardiologists have expressed a desire for more data to guide them in making prescribing decisions—namely, more real-world data rather than just clinical trial findings, and more data comparing NOACs to one another rather than to warfarin only.[39] The current analysis of NOACs vs. warfarin and NOACs vs. NOACs in a large US cohort of NVAF patients with prior bleeding could be useful to help inform clinical decision-making in this challenging patient population.
Limitations
Our findings should be interpreted in the context of a few limitations. As is the case with all retrospective observational studies, causal relationships cannot be determined between the study variables and outcomes of interest. The datasets analyzed in this study were limited to an extent, which could affect results: potential residual confounders, such as over-the-counter aspirin use, serum creatinine/creatinine clearance, and laboratory values, were unavailable, and their absence could introduce bias. Given that ICD, CPT, and HCPCS codes were used to identify the diagnoses and procedures, some variables in the datasets may lack clinical accuracy due to human data entry errors. Finally, the lack of laboratory information (e.g., lack of INR to determine time in therapeutic range) makes it difficult to assess the quality of warfarin control. Nevertheless, by including patients with potentially poor quality of warfarin treatment, this study may reflect real-world clinical practice.[40] It should also be noted that unobserved heterogeneity may exist across the 5 datasets used in this analysis. For the commercial datasets, although some of them include data from different insurance plans that do not overlap at the plan level, others are employer-based claims datasets which may contain duplicate patient records when pooled together. But the likelihood of duplicate observations is relatively low, researched to be 0.5%, and is not likely to have a significant impact on study results.[41] To avoid potential duplications the commercial datasets with Medicare data, patients with Medicare supplemental plans in MarketScan and PharMetrics data were excluded. For Optum and Humana data, beneficiaries aged ≥ 65 years are not covered in Medicare data and therefore do not have duplicates.
Conclusions
To our knowledge, this is the first real-world data analysis of stroke/SE and MB outcomes of NOACs vs. warfarin and vs. one another in a U.S. sample of NVAF patients with prior bleeding. The results indicate that treatment with NOACs was associated with similar or lower risk of stroke/SE and MB compared with warfarin and variable risk of stroke/SE and MB against each other.
Notes
There were 241 patients with an edoxaban treatment episode captured, which accounted for 0.1% of the sample.
References
Burdett P, Lip GYH (2020) Atrial Fibrillation in the United Kingdom: Predicting Costs of an Emerging Epidemic Recognising and Forecasting the Cost Drivers of Atrial Fibrillation-related costs. Eur Heart J Qual Care Clin Outcomes. https://doi.org/10.1093/ehjqcco/qcaa093
Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, Commerford P, Jansky P, Avezum A, Sigamani A, Damasceno A, Reilly P, Grinvalds A, Nakamya J, Aje A, Almahmeed W, Moriarty A, Wallentin L, Yusuf S, Connolly SJ, Registry R-LAF, Cohort Study I (2016) Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet 388:1161–1169. https://doi.org/10.1016/S0140-6736(16)30968-0
Lip GYH (2017) The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 14:627–628. https://doi.org/10.1038/nrcardio.2017.153
Bhat A, Khanna S, Chen HHL, Gupta A, Gan GCH, Denniss AR, MacIntyre CR, Tan TC (2021) Integrated Care in Atrial Fibrillation: A Road Map to the Future. Circ Cardiovasc Qual Outcomes 14:e007411. https://doi.org/10.1161/CIRCOUTCOMES.120.007411
Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Sung JH, Pak HN, Lee MH, Joung B, Lip GYH (2019) Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb Haemost. 119: 1695 – 703. https://doi.org/10.1055/s-0039-1693516
Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, Gumprecht J, Koziel M, Yang PS, Guo Y, Lip GYH, Proietti M (2021) Adherence to the ‘Atrial Fibrillation Better Care’ Pathway in Patients with Atrial Fibrillation: Impact on Clinical Outcomes-A Systematic Review and Meta-Analysis of 285,000 Patients. Thromb Haemost. https://doi.org/10.1055/a-1515-9630
Chao TF, Liu CJ, Liao JN, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chung FP, Chen TJ, Lip GY, Chen SA (2016) Use of Oral Anticoagulants for Stroke Prevention in Patients With Atrial Fibrillation Who Have a History of Intracranial Hemorrhage. Circulation 133:1540–1547. https://doi.org/10.1161/circulationaha.115.019794
Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY (2010) A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 138:1093–1100. https://doi.org/10.1378/chest.10.0134
Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, Flechsenhar J, Neugebauer H, Juttler E, Grau A, Palm F, Rother J, Michels P, Hamann GF, Huwel J, Hagemann G, Barber B, Terborg C, Trostdorf F, Bazner H, Roth A, Wohrle J, Keller M, Schwarz M, Reimann G, Volkmann J, Mullges W, Kraft P, Classen J, Hobohm C, Horn M, Milewski A, Reichmann H, Schneider H, Schimmel E, Fink GR, Dohmen C, Stetefeld H, Witte O, Gunther A, Neumann-Haefelin T, Racs AE, Nueckel M, Erbguth F, Kloska SP, Dorfler A, Kohrmann M, Schwab S, Huttner HB (2015) Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 313:824–836. https://doi.org/10.1001/jama.2015.0846
Li YG, Lip GYH (2018) Anticoagulation Resumption After Intracerebral Hemorrhage. Curr Atheroscler Rep 20:32. https://doi.org/10.1007/s11883-018-0733-y
Murphy MP, Kuramatsu JB, Leasure A, Falcone GJ, Kamel H, Sansing LH, Kourkoulis C, Schwab K, Elm JJ, Gurol ME, Tran H, Greenberg SM, Viswanathan A, Anderson CD, Schwab S, Rosand J, Shi FD, Kittner SJ, Testai FD, Woo D, Langefeld CD, James ML, Koch S, Huttner HB, Biffi A, Sheth KN (2018) Cardioembolic Stroke Risk and Recovery After Anticoagulation-Related Intracerebral Hemorrhage. Stroke 49:2652–2658. https://doi.org/10.1161/STROKEAHA.118.021799
Newman TV, Chen N, He M, Saba S, Hernandez I (2020) Effectiveness and Safety of Restarting Oral Anticoagulation in Patients with Atrial Fibrillation after an Intracranial Hemorrhage: Analysis of Medicare Part D Claims Data from 2010–2016. Am J Cardiovasc Drugs 20:471–479. https://doi.org/10.1007/s40256-019-00388-8
Qureshi W, Mittal C, Patsias I, Garikapati K, Kuchipudi A, Cheema G, Elbatta M, Alirhayim Z, Khalid F (2014) Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 113:662–668. https://doi.org/10.1016/j.amjcard.2013.10.044
Staerk L, Lip GY, Olesen JB, Fosbol EL, Pallisgaard JL, Bonde AN, Gundlund A, Lindhardt TB, Hansen ML, Torp-Pedersen C, Gislason GH (2015) Stroke and recurrent haemorrhage associated with antithrombotic treatment after gastrointestinal bleeding in patients with atrial fibrillation: nationwide cohort study. BMJ 351:h5876. https://doi.org/10.1136/bmj.h5876
Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY (2015) Restarting Anticoagulant Treatment After Intracranial Hemorrhage in Patients With Atrial Fibrillation and the Impact on Recurrent Stroke, Mortality, and Bleeding: A Nationwide Cohort Study. Circulation 132:517–525. https://doi.org/10.1161/CIRCULATIONAHA.115.015735
Proietti M, Romiti GF, Romanazzi I, Farcomeni A, Staerk L, Nielsen PB, Lip GYH (2018) Restarting oral anticoagulant therapy after major bleeding in atrial fibrillation: A systematic review and meta-analysis. Int J Cardiol 261:84–91. https://doi.org/10.1016/j.ijcard.2018.03.053
Li G, Lip GYH, Holbrook A, Chang Y, Larsen TB, Sun X, Tang J, Mbuagbaw L, Witt DM, Crowther M, Thabane L, Levine MAH (2019) Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: a systematic review and meta-analysis of observational studies. Eur J Epidemiol 34:173–190. https://doi.org/10.1007/s10654-018-0415-7
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM (2014) Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 383:955–962. https://doi.org/10.1016/S0140-6736(13)62343-0
Hohmann C, Hohnloser SH, Jacob J, Walker J, Baldus S, Pfister R (2019) Non-Vitamin K Oral Anticoagulants in Comparison to Phenprocoumon in Geriatric and Non-Geriatric Patients with Non-Valvular Atrial Fibrillation. Thromb Haemost. 119: 971 – 80. https://doi.org/10.1055/s-0039-1683422
Hohnloser SH, Basic E, Nabauer M (2019) Changes in Oral Anticoagulation Therapy over One Year in 51,000 Atrial Fibrillation Patients at Risk for Stroke: A Practice-Derived Study Thromb Haemost. 119: 882 – 93. https://doi.org/10.1055/s-0039-1683428
Kohsaka S, Katada J, Saito K, Jenkins A, Li B, Mardekian J, Terayama Y (2020) Safety and effectiveness of non-vitamin K oral anticoagulants versus warfarin in real-world patients with non-valvular atrial fibrillation: a retrospective analysis of contemporary Japanese administrative claims data. Open Heart 7:e001232. https://doi.org/10.1136/openhrt-2019-001232
Nielsen PB, Skjoth F, Sogaard M, Kjaeldgaard JN, Lip GYH, Larsen TB (2019) Non-Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Atrial Fibrillation Patients With Intracerebral Hemorrhage. Stroke 50:939–946. https://doi.org/10.1161/STROKEAHA.118.023797
Tsai CT, Liao JN, Chiang CE, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chung FP, Chao TF, Lip GYH, Chen SA (2020) Association of Ischemic Stroke, Major Bleeding, and Other Adverse Events With Warfarin Use vs Non-vitamin K Antagonist Oral Anticoagulant Use in Patients With Atrial Fibrillation With a History of Intracranial Hemorrhage. JAMA Netw Open 3:e206424. https://doi.org/10.1001/jamanetworkopen.2020.6424
Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, Luo X, Mardekian J, Friend K, Nadkarni A, Pan X, Baser O, Deitelzweig S (2018) Effectiveness and Safety of Oral Anticoagulants Among Nonvalvular Atrial Fibrillation Patients. Stroke 49:2933–2944. https://doi.org/10.1161/STROKEAHA.118.020232
Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA (2011) An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf 20:560–566. https://doi.org/10.1002/pds.2109
Thigpen JL, Dillon C, Forster KB, Henault L, Quinn EK, Tripodis Y, Berger PB, Hylek EM, Limdi NA (2015) Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes 8:8–14. https://doi.org/10.1161/CIRCOUTCOMES.113.000371
Chan YH, Yeh YH, Tu HT, Kuo CT, Chang SH, Wu LS, Lee HF, See LC (2017) Bleeding risk with dabigatran, rivaroxaban, warfarin, and antiplatelet agent in Asians with non-valvular atrial fibrillation. Oncotarget 8:98898–98917. https://doi.org/10.18632/oncotarget.22026
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107. https://doi.org/10.1002/sim.3697
Austin PC (2009) The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 33:1242–1258. https://doi.org/10.1002/sim.5984
Hernandez I, Zhang Y, Brooks MM, Chin PK, Saba S (2017) Anticoagulation Use and Clinical Outcomes After Major Bleeding on Dabigatran or Warfarin in Atrial Fibrillation. Stroke 48:159–166. https://doi.org/10.1161/STROKEAHA.116.015150
Kwon S, Lee SR, Choi EK, Lee E, Jung JH, Han KD, Cha MJ, Oh S, Lip GYH (2021) Non-Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial Fibrillation and Prior Gastrointestinal Bleeding. Stroke 52:511–520. https://doi.org/10.1161/STROKEAHA.120.030761
Lee SR, Choi EK, Kwon S, Jung JH, Han KD, Cha MJ, Oh S, Lip GYH (2020) Oral Anticoagulation in Asian Patients With Atrial Fibrillation and a History of Intracranial Hemorrhage. Stroke 51:416–423. https://doi.org/10.1161/STROKEAHA.119.028030
Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW (2018) Trends and Variation in Oral Anticoagulant Choice in Patients with Atrial Fibrillation, 2010–2017. Pharmacotherapy 38:907–920. https://doi.org/10.1002/phar.2158
Staerk L, Fosbol EL, Gadsboll K, Sindet-Pedersen C, Pallisgaard JL, Lamberts M, Lip GY, Torp-Pedersen C, Gislason GH, Olesen JB (2016) Non-vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: Temporal trends 2011–2015 in Denmark. Sci Rep 6:31477. https://doi.org/10.1038/srep31477
Kjerpeseth LJ, Ellekjaer H, Selmer R, Ariansen I, Furu K, Skovlund E (2017) Trends in use of warfarin and direct oral anticoagulants in atrial fibrillation in Norway, 2010 to 2015. Eur J Clin Pharmacol 73:1417–1425. https://doi.org/10.1007/s00228-017-2296-1
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW (2019) 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 74:104–132. https://doi.org/10.1016/j.jacc.2019.01.011
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, Group ESCSD (2021) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 42:373–498. https://doi.org/10.1093/eurheartj/ehaa612
Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, Lane DA, Ruff CT, Turakhia M, Werring D, Patel S, Moores L (2018) Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest. 154: 1121 – 201. https://doi.org/10.1016/j.chest.2018.07.040
Eek AK, Oie E, Granas AG (2018) Prescribing of NOACs has outnumbered warfarin: exploring how physicians choose anticoagulant treatments. Eur J Clin Pharmacol 74:323–330. https://doi.org/10.1007/s00228-017-2374-4
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L (2009) Committee R-LS, Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361:1139–1151. https://doi.org/10.1056/NEJMoa0905561
Saliba W (2018) Anticoagulation Management: Therapeutic Selection and Dosing. American College of Cardiology Middle East Conference. Jeddah, Saudi Arabia, 2018
Acknowledgements
Authorship. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, have read and given their approval for this version to be published.
Funding
This study was funded by Bristol Myers Squibb, Lawrenceville, NJ, and Pfizer, New York, NY.
Author information
Authors and Affiliations
Contributions
Conceptualization: all authors; Methodology: all authors; Formal Analysis and Investigation: Allison Keshishian; Writing – original draft preparation: Allison Keshishian; Writing – review and editing; all authors. Funding Acquisition: Amiee Kang, Xuemei Luo, Nipun Atreja, Yan Zhang, Patricia Schuler, Jenny Jiang; Resources: Huseyin Yuce; Supervision: Gregory Y.H. Lip, Steven Deitelzweig. All authors contributed to the conceptualization or design the work. All authors contributed to the data acquisition for the work. Allison Keshishian contributed to analyzing the data for the work. All authors contributed to interpreting the data for the work. Allison Keshishian drafted the work. All authors critically revised for important intellectual content. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Conflict of interest
Gregory Y. H. Lip and Steven Deitelzweig received research support from Pfizer and Bristol Myers Squibb. Allison Keshishian is employed by STATinMED Research, a paid consultant to Pfizer and Bristol Myers Squibb, in connection with the development of this manuscript. Amiee Kang, Nipun Atreja, Yan Zhang, Patricia Schuler, and Jenny Jiang are employees of Bristol Myers Squibb, a study sponsor. XL is an employee of Pfizer, a study sponsor. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lip, G.Y.H., Keshishian, A., Kang, A. et al. Effectiveness and safety of oral anticoagulants in non-valvular atrial fibrillation patients with prior bleeding events: a retrospective analysis of administrative claims databases. J Thromb Thrombolysis 54, 33–46 (2022). https://doi.org/10.1007/s11239-022-02660-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-022-02660-2