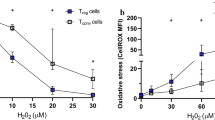
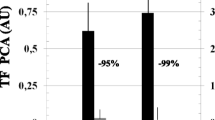
Abstract
Several studies have shown that T-cells might be involved in pathophysiology of acute coronary syndromes (ACS). Tissue factor (TF) plays a key role in ACS. Many evidences have indicated that some statins reduce TF expression in several cell types. However, literature about rosuvastatin and TF and about statins effects on T-cells is still scanty. Colchicine is an anti-inflammatory drug recently proven to have beneficial effects in ACS via unknown mechanisms. This study investigates the effects of colchicine and rosuvastatin on TF expression in oxLDL-activated T-cells. T-cells, isolated from buffy coats of healthy volunteers, were stimulated with oxLDL (50 µg/dL). T-cells were pre-incubated with colchicine (10 µM) or rosuvastatin (5 µM) for 1 h and then stimulated with oxLDL (50 μg/mL). TF gene (RT-PCR), protein (western blot), surface expression (FACS) and procoagulant activity (FXa generation assay) were measured. NF-κB/IκB axis was examined by western blot analysis and translocation assay. Colchicine and rosuvastatin significantly reduced TF gene, and protein expression and procoagulant activity in oxLDL stimulated T-cells. This effect was associated with a significant reduction in TF surface expression as well as its procoagulant activity. These phenomena appear modulated by drug effects on the transcription factor NF-kB. Rosuvastatin and colchicine prevent TF expression in oxLDL-stimulated T-cells by modulating the NF-κB/IκB axis. Thus, we speculate that this might be another mechanism by which these drugs exert benefic cardiovascular effects.






Similar content being viewed by others
References
Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ et al (2020) Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 41:2313–2330. https://doi.org/10.1093/eurheartj/ehz962
Di Pietro N, Formoso G, Pandolfi A (2016) Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc Pharmacol 84:1–7. https://doi.org/10.1016/j.vph.2016.05.013
Simionescu M (2007) Implications of early structural–functional changes in the endothelium for vascular disease. Arterioscler Thromb Vasc Biol 27:266–274. https://doi.org/10.1161/01.ATV.0000253884.13901.e4
Cimmino G, Loffredo FS, Morello A, D’Elia S, De Palma R, Cirillo P, Golino P (2017) Immune-inflammatory activation in acute coronary syndromes: a look into the heart of unstable coronary plaque. Curr Cardiol Rev 13:110–117. https://doi.org/10.2174/1573403X12666161014093812
Profumo E, Buttari B, Tosti ME, Tagliani A, Capoano R, D’Amati G, Businaro R, Salvati B, Rigano R (2013) Plaque-infiltrating T lymphocytes in patients with carotid atherosclerosis: an insight into the cellular mechanisms associated to plaque destabilization. J Cardiovasc Surg 54:349–357
Samson S, Mundkur L, Kakkar VV (2012) Immune response to lipoproteins in atherosclerosis. Cholesterol 2012:571846. https://doi.org/10.1155/2012/571846
Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6:508–519. https://doi.org/10.1038/nri1882
De Palma R, Del Galdo F, Abbate G, Chiariello M, Calabro R, Forte L, Cimmino G, Papa MF, Russo MG, Ambrosio G et al (2006) Patients with acute coronary syndrome show oligoclonal T-cell recruitment within unstable plaque: evidence for a local, intracoronary immunologic mechanism. Circulation 113:640–646. https://doi.org/10.1161/CIRCULATIONAHA.105.537712
De Palma R, Cirillo P, Ciccarelli G, Barra G, Conte S, Pellegrino G, Pasquale G, Nassa G, Pacifico F, Leonardi A et al (2016) Expression of functional tissue factor in activated T-lymphocytes in vitro and in vivo: a possible contribution of immunity to thrombosis? Int J Cardiol 218:188–195. https://doi.org/10.1016/j.ijcard.2016.04.177
Oesterle A, Laufs U, Liao JK (2017) Pleiotropic effects of statins on the cardiovascular system. Circ Res 120:229–243. https://doi.org/10.1161/CIRCRESAHA.116.308537
Yu D, Liao JK (2021) Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc Res. https://doi.org/10.1093/cvr/cvab032
Hilgendorff A, Muth H, Parviz B, Staubitz A, Haberbosch W, Tillmanns H, Holschermann H (2003) Statins differ in their ability to block NF-kappaB activation in human blood monocytes. Int J Clin Pharmacol Ther 41:397–401. https://doi.org/10.5414/cpp41397
Banfi C, Brioschi M, Lento S, Pirillo A, Galli S, Cosentino S, Tremoli E, Mussoni L (2011) Statins prevent tissue factor induction by protease-activated receptors 1 and 2 in human umbilical vein endothelial cells in vitro. J Thromb Haemost 9:1608–1619. https://doi.org/10.1111/j.1538-7836.2011.04366.x
Panes O, Gonzalez C, Hidalgo P, Valderas JP, Acevedo M, Contreras S, Sanchez X, Pereira J, Rigotti A, Mezzano D (2017) Platelet tissue factor activity and membrane cholesterol are increased in hypercholesterolemia and normalized by rosuvastatin, but not by atorvastatin. Atherosclerosis 257:164–171. https://doi.org/10.1016/j.atherosclerosis.2016.12.019
Ridker PM, Thuren T, Zalewski A, Libby P (2011) Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 162:597–605. https://doi.org/10.1016/j.ahj.2011.06.012
Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA et al (2013) Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 166:199–207. https://doi.org/10.1016/j.ahj.2013.03.018
Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T et al (2020) Colchicine in patients with chronic coronary disease. N Engl J Med 383:1838–1847. https://doi.org/10.1056/NEJMoa2021372
Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS et al (2019) Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 381:2497–2505. https://doi.org/10.1056/NEJMoa1912388
Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL (2013) Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 61:404–410. https://doi.org/10.1016/j.jacc.2012.10.027
Cimmino G, Tarallo R, Conte S, Morello A, Pellegrino G, Loffredo FS, Cali G, De Luca N, Golino P, Trimarco B et al (2018) Colchicine reduces platelet aggregation by modulating cytoskeleton rearrangement via inhibition of cofilin and LIM domain kinase 1. Vascul Pharmacol 111:62–70. https://doi.org/10.1016/j.vph.2018.09.004
Cirillo P, Taglialatela V, Pellegrino G, Morello A, Conte S, Di Serafino L, Cimmino G (2020) Effects of colchicine on platelet aggregation in patients on dual antiplatelet therapy with aspirin and clopidogrel. J Thromb Thrombol 50:468–472. https://doi.org/10.1007/s11239-020-02121-8
Cimmino G, Conte S, Morello A, Pellegrino G, Marra L, Cali G, Golino P, Cirillo P (2021) Colchicine inhibits the prothrombotic effects of oxLDL in human endothelial cells. Vasc Pharmacol 137:106822. https://doi.org/10.1016/j.vph.2020.106822
Cimmino G, Ibanez B, Vilahur G, Speidl WS, Fuster V, Badimon L, Badimon JJ (2009) Up-regulation of reverse cholesterol transport key players and rescue from global inflammation by ApoA-I(Milano). J Cell Mol Med 13:3226–3235. https://doi.org/10.1111/j.1582-4934.2008.00614.x
Cimmino G, Cirillo P, Conte S, Pellegrino G, Barra G, Maresca L, Morello A, Cali G, Loffredo F, De Palma R et al (2020) Oxidized low-density lipoproteins induce tissue factor expression in T-lymphocytes via activation of lectin-like oxidized low-density lipoprotein receptor-1. Cardiovasc Res 116:1125–1135. https://doi.org/10.1093/cvr/cvz230
Huggett JF (2020) The digital MIQE guidelines update: minimum information for publication of quantitative digital PCR experiments for 2020. Clin Chem 66:1012–1029. https://doi.org/10.1093/clinchem/hvaa125
Oeth P, Parry GC, Mackman N (1997) Regulation of the tissue factor gene in human monocytic cells. Role of AP-1, NF-kappa B/Rel, and Sp1 proteins in uninduced and lipopolysaccharide-induced expression. Arterioscler Thromb Vasc Biol 17:365–374. https://doi.org/10.1161/01.atv.17.2.365
Cirillo P, Conte S, Cimmino G, Pellegrino G, Ziviello F, Barra G, Sasso FC, Borgia F, De Palma R, Trimarco B (2017) Nobiletin inhibits oxidized-LDL mediated expression of tissue factor in human endothelial cells through inhibition of NF-kappaB. Biochem Pharmacol 128:26–33. https://doi.org/10.1016/j.bcp.2016.12.016
Wolf D, Ley K (2019) Immunity and inflammation in atherosclerosis. Circ Res 124:315–327. https://doi.org/10.1161/CIRCRESAHA.118.313591
Riksen NP, Stienstra R (2018) Metabolism of innate immune cells: impact on atherosclerosis. Curr Opin Lipidol 29:359–367. https://doi.org/10.1097/MOL.0000000000000539
Chistiakov DA, Orekhov AN, Bobryshev YV (2016) Immune-inflammatory responses in atherosclerosis: role of an adaptive immunity mainly driven by T and B cells. Immunobiology 221:1014–1033. https://doi.org/10.1016/j.imbio.2016.05.010
Schaftenaar F, Frodermann V, Kuiper J, Lutgens E (2016) Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol 27:209–215. https://doi.org/10.1097/MOL.0000000000000302
Cirillo P, Cimmino G, D’Aiuto E, Di Palma V, Abbate G, Piscione F, Golino P, De Palma R (2014) Local cytokine production in patients with acute coronary syndromes: a look into the eye of the perfect (cytokine) storm. Int J Cardiol 176:227–229. https://doi.org/10.1016/j.ijcard.2014.05.035
Gotsman I, Sharpe AH, Lichtman AH (2008) T-cell costimulation and coinhibition in atherosclerosis. Circ Res 103:1220–1231. https://doi.org/10.1161/CIRCRESAHA.108.182428
Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM (2000) Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101:2883–2888. https://doi.org/10.1161/01.cir.101.25.2883
Aukrust P, Otterdal K, Yndestad A, Sandberg WJ, Smith C, Ueland T, Oie E, Damas JK, Gullestad L, Halvorsen B (2008) The complex role of T-cell-based immunity in atherosclerosis. Curr Atheroscler Rep 10:236–243. https://doi.org/10.1007/s11883-008-0037-8
Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK (1995) T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA 92:3893–3897. https://doi.org/10.1073/pnas.92.9.3893
Santos-Gallego CG, Picatoste B, Badimon JJ (2014) Pathophysiology of acute coronary syndrome. Curr Atheroscler Rep 16:401. https://doi.org/10.1007/s11883-014-0401-9
Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT et al (2003) Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361:1149–1158. https://doi.org/10.1016/S0140-6736(03)12948-0
Scandinavian Simvastatin Survival Study Group (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344:1383–1389
Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T et al (2001) Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 285:1711–1718. https://doi.org/10.1001/jama.285.13.1711
Serruys PW, de Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, Branzi A, Bertolami MC, Jackson G, Strauss B et al (2002) Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 287:3215–3222. https://doi.org/10.1001/jama.287.24.3215
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG et al (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359:2195–2207. https://doi.org/10.1056/NEJMoa0807646
Karmaus PW, Shi M, Perl S, Biancotto A, Candia J, Cheung F, Kotliarov Y, Young N, Fessler MB (2019) Effects of rosuvastatin on the immune system in healthy volunteers with normal serum cholesterol. JCI Insight. https://doi.org/10.1172/jci.insight.131530
Chen LW, Lin CS, Tsai MC, Shih SF, Lim ZW, Chen SJ, Tsui PF, Ho LJ, Lai JH, Liou JT (2019) Pitavastatin exerts potent anti-inflammatory and immunomodulatory effects via the suppression of AP-1 signal transduction in human T cells. Int J Mol Sci. https://doi.org/10.3390/ijms20143534
Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S (2015) Colchicine: old and new. Am J Med 128:461–470. https://doi.org/10.1016/j.amjmed.2014.12.010
Leung YY, Yao Hui LL, Kraus VB (2015) Colchicine-update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 45:341–350. https://doi.org/10.1016/j.semarthrit.2015.06.013
Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C et al (2015) 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 36:2921–2964. https://doi.org/10.1093/eurheartj/ehv318
Martinez GJ, Robertson S, Barraclough J, Xia Q, Mallat Z, Bursill C, Celermajer DS, Patel S (2015) Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J Am Heart Assoc 4:e002128. https://doi.org/10.1161/JAHA.115.002128
Yamamoto Y, Gaynor RB (2004) IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem Sci 29:72–79. https://doi.org/10.1016/j.tibs.2003.12.003
Mackman N (1997) Regulation of the tissue factor gene. Thromb Haemost 78:747–754
de Winther MP, Kanters E, Kraal G, Hofker MH (2005) Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol 25:904–914. https://doi.org/10.1161/01.ATV.0000160340.72641.87
Aggarwal BB, Takada Y, Shishodia S, Gutierrez AM, Oommen OV, Ichikawa H, Baba Y, Kumar A (2004) Nuclear transcription factor NF-kappa B: role in biology and medicine. Indian J Exp Biol 42:341–353
Cao Y, Zhou X, Liu H, Zhang Y, Yu X, Liu C (2013) The NF-kappaB pathway: regulation of the instability of atherosclerotic plaques activated by Fg, Fb, and FDPs. Mol Cell Biochem 383:29–37. https://doi.org/10.1007/s11010-013-1751-2
Liuzzo G, Santamaria M, Biasucci LM, Narducci M, Colafrancesco V, Porto A, Brugaletta S, Pinnelli M, Rizzello V, Maseri A et al (2007) Persistent activation of nuclear factor kappa-B signaling pathway in patients with unstable angina and elevated levels of C-reactive protein evidence for a direct proinflammatory effect of azide and lipopolysaccharide-free C-reactive protein on human monocytes via nuclear factor kappa-B activation. J Am Coll Cardiol 49:185–194. https://doi.org/10.1016/j.jacc.2006.07.071
Wilson SH, Best PJ, Edwards WD, Holmes DR Jr, Carlson PJ, Celermajer DS, Lerman A (2002) Nuclear factor-kappaB immunoreactivity is present in human coronary plaque and enhanced in patients with unstable angina pectoris. Atherosclerosis 160:147–153. https://doi.org/10.1016/s0021-9150(01)00546-9
Ritchie ME (1998) Nuclear factor-kappaB is selectively and markedly activated in humans with unstable angina pectoris. Circulation 98:1707–1713. https://doi.org/10.1161/01.cir.98.17.1707
Cirillo P, Ziviello F, Pellegrino G, Conte S, Cimmino G, Giaquinto A, Pacifico F, Leonardi A, Golino P, Trimarco B (2015) The adipokine apelin-13 induces expression of prothrombotic tissue factor. Thromb Haemost 113:363–372. https://doi.org/10.1160/TH14-05-0451
Cirillo P, Di Palma V, Maresca F, Pacifico F, Ziviello F, Bevilacqua M, Trimarco B, Leonardi A, Chiariello M (2012) The adipokine visfatin induces tissue factor expression in human coronary artery endothelial cells: another piece in the adipokines puzzle. Thromb Res 130:403–408. https://doi.org/10.1016/j.thromres.2012.06.007
Calabro P, Cirillo P, Limongelli G, Maddaloni V, Riegler L, Palmieri R, Pacileo G, De Rosa S, Pacileo M, De Palma R et al (2011) Tissue factor is induced by resistin in human coronary artery endothelial cells by the NF-kB-dependent pathway. J Vasc Res 48:59–66. https://doi.org/10.1159/000318775
Dichtl W, Dulak J, Frick M, Alber HF, Schwarzacher SP, Ares MP, Nilsson J, Pachinger O, Weidinger F (2003) HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 23:58–63. https://doi.org/10.1161/01.atv.0000043456.48735.20
Funding
The present work have received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has had a relationship with the industry within the past 2 years that might pose a conflict of interest in connection with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cirillo, P., Conte, S., Pellegrino, G. et al. Effects of colchicine on tissue factor in oxLDL-activated T-lymphocytes. J Thromb Thrombolysis 53, 739–749 (2022). https://doi.org/10.1007/s11239-021-02585-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02585-2