Abstract
Two species of sea lice are reported from the golden snapper Lutjanus johnii (Bloch) in Australian waters. One was represented by chalimus larvae, adult males and extremely slender females in which the genital complex is scarcely wider than the fourth pedigerous somite. These females are adult as they carry paired spermatophores and are identified as Caligus dussumieri Rangnekar, 1957 on the details of their appendages. Caligus dussumieri was formerly placed in the genus Sinocaligus Shen, 1957 but the characters supporting the validity of this genus are not robust, so it is here proposed to treat it as a junior subjective synonym of Caligus and transfer its species as: Caligus formicoides Redkar, Rangnekar & Murti, 1949, Caligus dussumieri Shen, 1957, Caligus caudatus (Gnanamuthu, 1950) new combination and Caligus timorensis (Izawa, 1995) new combination. All these species can be placed in the C. bonito-species group within Caligus. Caligus rivulatus Pilla, Vankara & Chikkam, 2012 is recognized as a junior subjective synonym of C. dussumieri. A new species, C. auriolus n. sp. is also described and this is placed in the C. diaphanus species-group. A key to species of this species-group is provided which indicates that C. auriolus n. sp. is most closely related to C. stromatei Krøyer, 1863 but the latter can be distinguished by the slender abdomen of the female and by the more complex myxal process on the maxilliped in the male.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Caligidae Burmeister, 1835 is the most species rich family of parasitic copepods and, since caligid sea lice are one of the most important health hazards for farmed marine finfish (Johnson et al., 2004), it is of enormous commercial importance (Boxaspen et al., 2022). Boxshall (2018) reviewed historic data on caligids in Australian waters and found records of 69 species. He also reported another 16 species from Australia for the first time and described 13 new species from Moreton Bay, Queensland. The total number of caligid species known from Australian marine fishes after Boxshall’s (2018) contribution was 98. Since then, an additional species, Lepeophtheirus spinifer Kirtisinghe, 1937, has been reported from northern Australia (Diggles et al., 2021). Here we report on two further species, both taken from golden snapper, Lutjanus johnii, caught at various localities around the coast of the Northern Territory and Western Australia.
In addition to these caligids, copepods representing the families Lernanthropidae and Hatschekiidae were collected from the gills of the golden snapper. The two species of Lernanthropidae have been included in a separate larger study (Boxshall et al., 2020), while the hatschekiids have yet to be examined.
Materials and Methods
Fish were collected by hook and line from several locations along the northern Australia coastline from Camden Sound in Western Australia to Townsville in northern Queensland. All fish were frozen prior to analysis: following defrosting, the gills and pharyngeal teeth plates were removed, separated and washed in water; gill arches and pharyngeal teeth plates were examined separately for the presence of parasites; the external surface of fish was not examined. The supernatant for the wash was removed and the detritus examined under a dissecting microscope. Individual fish were given DPIF (Department of Primary Industries and Fisheries) numbers.
The copepods were preserved in 70% ethyl alcohol. Prior to examination the specimens were cleared in lactic acid for at least 2 h and mounted on glass slides as temporary preparations. Limbs were dissected where necessary to observe fine details. Measurements were made using an ocular micrometer and drawings were made using a drawing tube on a Leitz Diaplan microscope equipped with differential interference contrast. Morphological terminology follows Boxshall (1990a) and Huys & Boxshall (1991); host fish names have been updated according to FishBase (Froese & Pauly, 2022).
The holotype of the new species is deposited in the collection of the Museum and Art Gallery of the Northern Territory (MAGNT) in Darwin; paratypes or voucher specimens of both species are deposited in the MAGNT and in the Natural History Museum, London.
Systematics
Caligus dussumieri Rangnekar, 1957
Syn: Sinocaligus dussumieri (Rangnekar, 1957)
Pseudopetalus dussumieri (Rangnekar, 1957)
Caligus rivulatus Pilla, Vankara & Chikkam, 2012 new synonym
Host: Lutjanus johnii (Bloch)
Material examined: 6♀♀, 9♂♂ and 8 chalimus stages: 1♀, 1♂, 1 chalimus from DPIF 2297, Lorna Shoal, Northern Territory (12° 22.01′S 130° 17.37′E) on 05.12.2013 (MAGNT Reg.No. Cr019550); 1♂ from DPIF 2157, Darwin Harbour, Northern Territory (12° 39.02′S 130° 58.00′E) on 28.08.2013 (MAGNT Reg. No. Cr019551); 1♂ from DPIF 2268, Darwin Harbour, NT on 13.10.2013 (MAGNT Reg. No. Cr019552); 1♀ (damaged) from DPIF 1485, Melville Island, Northern Territory (11° 44.46′S 131° 16.89′E) on 23.08.2012 (MAGNT Reg. No. Cr019553); 1♀, 2♂♂, 2 chalimus from DPIF 2377, Nicoll Island, Northern Territory (13° 28.30′S 136° 16.64′E) on 10.12.2013 (MAGNT Reg. No. Cr019554); Material in NHM, London: 1 chalimus from DPIF 2590, Lorna Shoal, NT on 26.03.2014; 2 chalimus (dried) from DPIF 2270, Darwin Harbour, NT on 13.10.2013; 1♀, 1 chalimus from DPIF 2606, Elcho Island, Northern Territory (11° 55.13′S 135° 53.61′E) on 16.04.2014; 1♂ from DPIF 2372, Nicoll Island, NT on 02.12.2013; 1 chalimus from DPIF 2378, Groote Eylandt, NT on 13.10.2013; 2♂♂ from DPIF 2394, Camden Sound, Western Australia (16° 11.52′S 124° 32.52′E) on 11.09.2013; 1♀, 1♂ from DPIF 2391, Ord River, Camden Sound, WA on 11.09.2013; 1♀ from DPIF 2395, Ord River, Camden Sound, WA on 11.09.2013, NHMUK Reg. Nos. 2022.189-197.
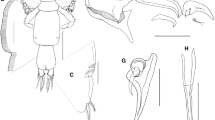
Description: Adult female (Fig. 1A) mean body length 3.28 mm (range 3.05 to 3.65 mm), including caudal rami (based on 5 specimens). Cephalothorax about 1.2 times longer than wide; comprising about 46% of total body length. Free margin of thoracic portion of dorsal cephalothoracic shield extending posteriorly beyond rear margins of lateral portions. Large lunules present ventrally on frontal plates. Genital complex elongate, scarcely wider than fourth pedigerous somite, about 2.3 times longer than wide (0.87 x 0.38 mm); with near-parallel lateral margins; fifth legs located on lateral margins (Fig. 1B). Genital complex about 1.2 times longer than abdomen. Abdomen elongate, 1-segmented; about 2.6 times longer than wide (0.70 x 0.27 mm) (Fig. 1B); carrying paired caudal rami distally; anal slit terminal. Caudal rami with parallel sides, longer than wide. Each ramus armed with 3 long plumose setae on distal margin, short hirsute seta at inner distal angle, spiniform, hirsute seta sub-distally on outer margin, plus minute seta located just ventral to outer distal seta (not visible in Fig. 1B).
Antennule (Fig. 1C) 2-segmented; large proximal segment with 25 plumose setae arrayed along anteroventral surface and 2 setae located dorsally; distal segment bearing 12 elements (10 setae plus 2 aesthetascs) around apex, plus isolated seta on posterior margin. Antenna (Fig. 1D) comprising proximal segment bearing blunt-tipped, posteriorly-directed spinous process; middle segment subrectangular, unarmed; terminal segment forming weakly curved claw bearing short spine proximally, and minute seta on anterior margin. Postantennal process (Fig. 1D) weakly curved; ornamented with 2 bi-sensillate papillae on basal part and uni-sensillate papilla on adjacent ventral cephalothoracic surface.
Mandible of typical stylet-like structure, with 12 marginal teeth (not figured). Maxillule (Fig. 2A) comprising anterior papilla bearing 3 unequal, naked setae and posterior, tine-like process. Maxilla 2-segmented (Fig. 2B), comprising elongate, unarmed syncoxa and basis: basis bearing membranous subapical flabellum on anterior margin, and terminating in 2 subequal claw-like elements (calamus and canna). Calamus longer than canna, ornamented with strips of serrated membrane arranged obliquely around surface; canna ornamented with bilateral strips of serrated membrane. Maxilliped subchelate (Fig. 2C); proximal segment unarmed, with slight swellings on myxal surface; distal subchela with apical claw separated from proximal segmental part by incomplete suture; small seta present near concave margin.
Sternal furca (Fig. 2D) with divergent, pointed tines.
First swimming leg (Fig. 2E) with slender intercoxal sclerite; sympod with inner and outer plumose setae derived from basis; endopod represented by unarmed conical process on posterior margin of basis. Exopod short, robust, 2-segmented; directed laterally and forming main axis of leg; first segment broad, only about 1.5 times longer than wide and armed with small outer (anterior) spine and ornamented with row of setules along part of posterior margin; second segment short, 1.3 times longer than wide, armed with 3 long plumose setae along posterior margin, each ornamented with stout spinules laterally and slender pinnules medially; distal margin with 4 elements as follows: spine 1 (anterior-most) longest; spine 2 longer than spine 3, each with accessory process; seta 4 unilaterally plumose, longer than spine 1 and about as long as segment.
Second leg (Fig. 2F) biramous, with flattened protopodal segments and 3-segmented rami. Coxae of leg pair joined by intercoxal sclerite bearing marginal membrane posteriorly. Coxa with plumose seta posteriorly and surface sensilla. Basis armed with outer naked seta; ornamented with surface sensilla, marginal membrane posteriorly, and flap of membrane anteriorly, reflexed back over dorsal surface of segment. Exopodal segment 1 with long, straight, outer spine extending obliquely across ventral surface of segments 2 and 3, also armed with inner plumose seta and bearing flap of membrane anteriorly, reflexed back over dorsal surface of segment; segment 2 with short outer spine aligned parallel with longitudinal axis of ramus, and inner plumose seta; segment 3 with 2 outer spines, proximal spine small, distal spine with large flap of membrane; apical spine with marginal membrane laterally and pinnules medially, and 5 inner plumose setae. Endopodal segment 1 armed with inner plumose seta and ornamented with few slender spinules at outer distal angle; segment 2 ornamented with conspicuous denticles along outer margin, and bearing 2 inner plumose setae; segment 3 with 6 plumose setae.
Third leg pair forming flattened plate (apron) closing posterior part of cephalothoracic sucker as typical for genus. Leg (Fig. 2G) fused to plate-like intercoxal sclerite ornamented with marginal membrane posteriorly. Protopodal part flattened, bearing inner plumose seta at junction with intercoxal plate, and outer plumose seta located dorsally near base of exopod; single sensillae located adjacent to inner coxal seta and adjacent to origin of endopod; ornamented with strip of membrane along posterior margin medial to endopod and along lateral margin anterior to exopod. Exopod 3-segmented; first segment lacking inner seta, armed with weakly curved outer spine directed over ventral surface of ramus, spine ornamented with bilateral strips of membrane; second segment with small outer spine and inner plumose seta; third with 3 outer spines and 4 inner plumose setae; outer margins of segments 2 and 3 ornamented with slender setules. Endopod 2-segmented; first segment forming velum ornamented with row of fine setules along free margin and armed with inner plumose seta; compound distal segment with 6 setal elements increasing in length from outermost to innermost.
Fourth leg (Fig. 2H) 3-segmented, comprising slender protopodal segment and 2-segmented exopod: protopodal segment armed with plumose seta distally; first exopodal segment armed with slender outer spine; second with 1 lateral plus 3 distal spines; apical spine slightly longer than middle spine; middle and outer spines of similar length; each spine with pecten at base.
Fifth legs located posterolaterally on genital complex (Fig. 1B); each fifth leg comprising anterior process bearing short plumose seta (representing outer protopodal seta) and inner exopodal process armed with 2 plumose setae.
Adult male (Fig. 1E) mean body length 3.02 mm (range 2.42 to 3.75 mm). including caudal rami (based on 10 specimens). Cephalothorax as in female. Genital complex (Fig. 3A) 1.3 times longer than wide (0.61 x 0.48 mm); with weakly convex lateral margins. Abdomen 2-segmented; first segment longer than wide (0.27 mm x 0.24 mm), second segment about 1.4 times longer than first, and about 1.6 times longer than wide (0.38 x 0.23 mm); carrying paired caudal rami posteriorly as in female.
Caligus dussumieri Rangnekar, 1957, adult male. A, fourth pedigerous somite, genital complex and abdomen, dorsal; B, fifth legs and genital apertures, ventral; C, antenna; D, post-antennary process; E, maxilliped; F, exopod of leg 2; G, first and second endopodal segments of leg 2 showing ornamentation; H, leg 4. Scale bars: A, 500 µm, B, E, F, H, 200 µm, C, D, G, 100 µm.
Antennule, mandible, maxillule and maxilla as in female. Antenna modified (Fig. 3C); first segment elongate; second segment reflexed, elongate, bearing corrugated adhesion pads ventrally in distal part and anteriorly in proximal part; distal segment forming short flattened claw, armed with 2 setae proximally. Postantennal process (Fig. 3D) more strongly curved than in female; ornamented with sensillate papillae.
Maxilliped (Fig. 3E) with 2 processes on myxal margin of proximal segment; proximal process tooth-like, distal process larger, with truncate apex, opposing tip of subchela.
Leg 1 as in female. Leg 2 with outer spine on first exopodal segment less well developed than in female (Fig. 3F); spine on second segment directed obliquely across surface of ramus; endopod segment 2 with slender spinules along outer margin rather than robust denticles (Fig. 3G). Leg 3 as in female. Leg 4 (Fig. 3H) similar to female but apical spine slightly longer relative to middle and outer distal spines.
Leg 5 (Fig. 3B) represented by plumose, outer protopodal seta originating on papilla on somite surface and 2 plumose setae on inner papilla representing exopod. Sixth leg represented by plate closing off genital opening armed with 1 seta and 1 short spine on outer distal corner of genital operculum.
Remarks: The adult females collected from Lutjanus johnii had an extremely long and slender genital complex and abdomen (Fig. 1A, B). These females carried paired spermatophores and are identified as adults since female copepods become sexually receptive only after the final, definitive moult (Boxshall, 1990b). The extreme narrowness of the genital complex exhibited by these females is unique in Caligus, however, detailed examination of their appendages revealed a close resemblance to Caligus biseriodentatus Shen, 1957 and to Sinocaligus dussumieri (Rangnekar, 1957).
Caligus biseriodentatus was recognized as a member of the Caligus bonito species-group by Boxshall (2018), based on its possession of a 2-segmented exopod on leg 4 bearing 4 spines on the compound distal segment, combined with the presence of 3 plumose setae on the posterior margin of the distal exopodal segment of leg 1 in the female, plus the ornamentation of large denticles along the outer margin of the second endopodal segment of leg 2. The Australian material from L. johnii shares all of these features (as do all species currently placed in Sinocaligus), but can be distinguished from C. biseriodentatus by the length of the plumose setae on the posterior margin of the distal exopodal segment of leg 1. These setae are well developed and longer than seta 4 in the Australian material but are markedly shorter than seta 4 in female of C. biseriodentatus and are even further reduced in the male (Shen, 1957; Cressey & Cressey, 1980; Boxshall, 2018). In addition, the first exopodal segment of leg 4 is ornamented with conspicuous surface spinules in the female of C. biseriodentatus (see Cressey & Cressey, 1980; Pillai, 1985 (as C. auxisi); Boxshall, 2018) but the Australian material from L. johnii lacks such ornamentation.
Sinocaligus dussumieri was originally described, as Caligus dussumieri, based on a single ovigerous female found on the inside surface of the operculum of a clupeiform fish, Dussumieria acuta Valenciennes, 1847, caught off Mumbai (Rangnekar, 1957). Pillai (1968) redescribed the species, based on two females collected from the gills of D. elopsoides Bleeker, 1849 (as D. hasseltii Bleeker), and transferred it to the genus Pseudopetalus Pillai, 1962 as P. dussumieri (Rangnekar, 1957). However, as Boxshall & Montú (1997) noted, Pseudopetalus is a junior synonym of Sinocaligus Shen, 1957. In their major review of the family Caligidae, Dojiri & Ho (2013) accepted S. dussumieri as a valid species of Sinocaligus. The redescription of S. dussumieri by Pillai (1968) revealed several distinctive features of the swimming legs of this caligid: leg 1 has unusually short but broad exopodal segments and each of the plumose setae on the posterior margin of the distal segment is longer than seta 4 and is ornamented with spinules along its lateral margin; the first exopodal segment of leg 2 carries a distinctive, elongate outer spine with a spatulate tip; the proximal outer margin spine on the third exopodal segment of leg 2 is small and the distal spine on the same margin is ornamented with a large flap of membrane. These characteristics are all shared with our material from golden snapper and there is close agreement in almost all of the other appendages. The only exception is the female maxilliped, which Pillai (1985) showed as bearing a tapering process. No process was figured by Rangnekar (1957) and none was present in our Australian material. The process shown by Pillai (1985: Fig. 132E) is not in the normal position for a myxal process, i.e. opposing the tip of the claw, but it was also mentioned by Pillai (1968) and requires further investigation.
The above comparisons are focused on the detailed similarities in the appendages, however, there is an apparent major difference between the Australian material from L. johnii and the Indian material described by Rangnekar (1957) and Pillai (1968, 1985) from Dussumieria species, and that is the shape of the female body. The ovigerous females found by Rangnekar (1957) and Pillai (1968) all have an elongate and somewhat swollen genital complex and a laterally expanded abdomen. It is, largely, the laterally expanded abdomen that has been used as a generic level character to support the validity of the genus Sinocaligus (see Dojiri & Ho, 2013, for discussion). Variability in shape is apparent even between these ovigerous females: the female illustrated by Rangnekar (1957: Fig. 2a) has a genital complex with a slender anterior part (comprising 25% of the total length) and the abdomen is about 3.5 times longer than wide, whereas in Pillai’s (1968) females the slender anterior part is short and the abdomen is only about 1.8 times longer than wide (Pillai, 1968). The females from L. johnii are not ovigerous but they are adult and have mated as they carry paired spermatophores. It seems likely that the females of this caligid undergo a post-mating metamorphosis resulting in a major lateral expansion of both the genital complex and the abdomen. Such a post-mating metamorphosis in adult females is widespread in caligids (Boxshall & Özak, 2022). The extremely slender females figured here represent the immediate post-mating morphology while the laterally expanded ovigerous female figured by Pillai (1968) represents the fully metamorphosed adult. The adult figured by Rangnekar (1957) is also ovigerous but shows a lesser state of expansion.
Given the numerous detailed similarities between their appendages, we identify this material as conspecific with Sinocaligus dussumieri (originally described as Caligus dussumieri), and we infer that the differences in shape of the genital complex and abdomen are indicative of the state of development in the post-mating metamorphosis. Dojiri & Ho (2013) also noted variation in the shape of the female abdomen between typical Sinocaligus formicoides formicoides (Redkar, Rangnekar & Murti, 1949) and its variety S. formicoides denticulatus (Shen, 1957). Material from Hainan Island in the South China Sea described by Shen (1957) possessed a wide abdomen whereas the material from India had a slender spindle-shaped abdomen (Redkar, Rangnekar & Murti, 1949; Pillai, 1962). The females examined by Dojiri & Ho (2013) had an abdomen somewhat intermediate between these two states and they interpreted this variation as plasticity. We infer that this plasticity is largely a developmental phenomenon, with the lateral expansion of the abdomen becoming more pronounced in older adult females.
The discovery of the new material raises serious questions concerning the validity of the genus Sinocaligus. The main features distinguishing Sinocaligus from Caligus Müller, 1785 are the aliform lateral expansions on the abdomen (Dojiri & Ho, 2013), although their phylogenetic analysis also scored the presence of 7 caudal setae on the caudal ramus and the presence of 25 or 26 setae on the proximal segment of the antennule (Dojiri & Ho, 2013: Table XXIII). These last two features are doubtful. Their figure of the caudal ramus (Dojiri & Ho, 2013 Fig. 138d) showed only 5 setae but with 2 small cuticular markings which they interpreted as missing setae. Although the possession of 7 caudal setae is the ancestral state of the Copepoda (Huys & Boxshall, 1991), only 6 caudal setae is the maximum number found in any caligid. Since the caudal rami carry also sensory sensillae in some caligids (which also leave a similar marking in the cuticle when detached), we regard the evidence supporting the presence of 7 caudal setae in Sinocaligus as extremely weak. Similarly, the apparently reduced setal count on the first antennulary segment is not a robust character. The great majority of caligids carry 27 setae (25 anteroventral and 2 dorsal) on this segment but the more ventrally located setae can be small and densely packed, so observations can be difficult. The Australian material from L. johnii has the typical 6 caudal setae (one of them minute) and has 27 (25 + 2) setae on the first antennulary segment as found in the great majority of Caligus species. Both Sinocaligus caudatus (Gnanamuthu, 1950) and Sinocaligus timorensis (Izawa, 1995) also possess only 6 caudal setae (Gnanamuthu, 1950; Izawa, 1995). The number of setae on the first antennulary segment is 20 in the former and 26 in the latter, but neither description mentions any dorsal setae and both therefore seem unreliable.
The remaining character used to justify the generic status of Sinocaligus is the lateral expansion of the abdomen of the female, but our new evidence indicates that the expansion of the genital complex and of the abdomen is a late developmental phenomenon. The males exhibit no features that would differentiate them from a typical Caligus male. We, therefore, propose to treat the genus Sinocaligus as a junior subjective synonym of Caligus and transfer all of its species: Sinocaligus formicoides (Redkar, Rangnekar & Murti, 1949) returns to its original combination as Caligus formicoides Redkar, Rangnekar & Murti, 1949 and Sinocaligus dussumieri (Shen, 1957) returns to its original combination as Caligus dussumieri Shen, 1957. Sinocaligus caudatus (Gnanamuthu, 1950) becomes Caligus caudatus (Gnanamuthu, 1950) new combination and Sinocaligus timorensis (Izawa, 1995), originally established as Pseudopetalus timorensis Izawa, 1995, becomes Caligus timorensis (Izawa, 1995) new combination. All of these species can be accommodated within the Caligus bonito species-group to which C. biseriodentatus belongs. Interestingly, the original female of C. biseriodentatus illustrated by Shen (1957: fig. 114) has the same very slender genital complex and abdomen and is presumably at the same pre-metamorphic phase.
We also note here an additional new synonymy. Pilla et al. (2012) described a Caligus species collected from the body surface of Lutjanus rivulatus Cuvier, 1828 caught off the Visakhapatnam coast, India and considered it to be a new species for which they proposed the name Caligus rivulatus. Unfortunately, their publication was in an on-line only journal and neither the publication nor the proposed new name was registered with ZooBank or given an LSID number, and thus this is not a valid publication.
Caligus rivulatus is based on seven specimens which were all considered to be males by Pilla et al. (2012). However, the illustrated specimen is a female, as indicated by the slender subchelate form of the antenna, the lack of myxal processes on the maxilliped, and the unsegmented state of the abdomen. The slender form of the genital complex and abdomen indicate that this female is pre-metamorphic but it is adult as indicated by the presence of spermatophores which are shown with dotted lines on Pilla et al.’s dorsal habitus figure. Pilla et al.’s (2012) figures of the appendages of C. rivulatus show: leg 1 carries 3 plumose setae on the posterior margin of the distal exopodal segment, leg 4 is 3-segmented with a 2-segmented exopod bearing I, IV spines, and the outer margin of the second endopodal segment of leg 2 is ornamented with large denticles. This combination of character states is shared by members of the Caligus bonito-group.
Caligus rivulatus shares the same distinctive setation pattern for female leg 1 with C. dussumieri and C. biseriodentatus: spines 1 to 3 on the distal exopodal segment decrease in size from outer to inner, spines 2 and 3 each bear an accessory process, seta 4 is longer than spine 1 and longer than the segment, and the 3 plumose setae on the posterior margin are each ornamented with stout spinules laterally. However, C. rivulatus also possesses the same distinctive elongate outer margin spine with a spatulate tip, as found on the first exopodal segment of leg 2 in female C. dussumieri. In view of this and the numerous other similarities we make C. rivulatus Pilla, Vankara & Chikkam, 2012 available here and also recognize it as a junior subjective synonym of Caligus dussumieri Shen, 1957. The body length given for C. rivulatus by Pilla et al. (2012) was 2.36 to 3.12 mm, which overlaps with that of the pre-metamorphic material of C. dussumieri reported here (3.05 – 3.65 mm).
Caligus auriolus n. sp .
Type Host: Lutjanus johnii (Bloch)
Type Locality: Lorna Shoal, near Darwin, Northern Territory (12° 22.008′S 130° 17.366′E).
Type Material: Holotype female deposited in the collections of the MAGNT (Reg. No. Cr019547) plus 2♀♀ paratypes (Reg. No. Cr019548) and 5♂♂ paratypes (Reg. No. Cr019549); remaining 3♀♀, 9♂♂ and 13 damaged specimens and chalimus stages in NHM, London (NHMUK 2022.179-188).
Material Examined: Holotype ♀ from DPIF 2192, Lorna Shoal, NT (12° 22.01′S 130° 17.37′E) on 18.09.2013; 5 paratype ♂♂, 1 chalimus from DPIF 2573, Lorna Shoal, NT on 26.03.2014; 2 paratype ♀♀ from DPIF 2590, Lorna Shoal, NT on 20.03.2014; 2 paratype ♀♀, 2 paratype ♂♂, 2 chalimus from DPIF 2589, Lorna Shoal, NT on 10.03.2014; 2 paratype ♂♂ from DPIF 2590, Lorna Shoal, NT on 20.03.2014; 1 paratype ♂ from DPIF 2573, Lorna Shoal, NT on 26.03.2014; 1 chalimus from DPIF 1918, Lorna Shoal, NT on 28.06.2013; 1 paratype ♂, 3 chalimus from DPIF 2602, Elcho Island, Northern Territory (11° 55.13′S 135° 53.61′E) on 16.04.2014; 1 chalimus from DPIF 2620, Elcho Island, NT on 16.04.2014; 2 paratype ♀♀, 7 incomplete and chalimus stages from DPIF 2378, Nicoll Island, Northern Territory (13° 28.30′S 136° 16.64′E) on 10.12.2013; 1 paratype ♂ from DPIF 2377, Nicoll Island, NT on 10.12.2013; 1 paratype ♂ from DPIF 2374 Nicoll Island, NT on 02.12.2013.
Etymology: The specific name auriolus comes from the Latin, meaning golden, and refers to the common name of the host, the golden snapper.
Description: Adult female (Fig. 4A) mean body length 4.18 mm (range 3.95 to 4.49 mm), including caudal rami (based on 5 specimens). Cephalothorax slightly longer than wide; comprising about 55% of total body length. Free margin of thoracic portion of dorsal cephalothoracic shield extending posteriorly beyond rear margins of lateral portions. Small lunules present ventrally on frontal plates. Genital complex about 1.2 times longer than wide (0.89 x 0.75 mm); with strongly convex lateral margins; fifth legs located on lateral margins (Fig. 4B). Genital complex about 1.35 times longer than abdomen. Abdomen elongate, 1-segmented; about 1.5 times longer than wide (0.66 x 0.45 mm) (Fig. 4B); anterior part of abdomen with transversely striated integument; carrying paired caudal rami distally; anal slit terminal. Caudal rami with parallel sides, longer than wide. Each ramus armed with 3 long plumose setae on distal margin, short hirsute seta at inner distal angle, spiniform, hirsute seta sub-distally on outer margin, plus small seta located just ventral to outer distal seta.
Caligus auriolus n. sp., adult female. A, habitus, dorsal; B, genital complex and abdomen with spermatophores attached, dorsal; C, antenna and postantennary process in situ; D, mandible; E, maxillule; F, maxilla; G, maxilliped; H, sternal furca. Scale bars: A, 1.0 mm, B, 500 µm, C-E, H, 100 µm, F, G, 200 µm.
Antennule (not figured) typical for genus: comprising large proximal segment with 25 plumose setae arrayed along anteroventral surface and 2 setae located dorsally; distal segment bearing 12 elements (10 setae plus 2 aesthetascs) around apex, plus isolated seta on posterior margin. Antenna (Fig. 4C) comprising proximal segment lacking any posterior process; middle segment subrectangular, unarmed; terminal segment forming curved claw bearing short spine proximally and small seta on anterior margin. Postantennal process (Fig. 4C) reduced, with short tine and ornamented with 2 multi-sensillate papillae on basal part; similar multisensillate papilla present on adjacent ventral cephalothoracic surface.
Mandible (Fig. 4D) stylet-like, with 12 marginal teeth. Maxillule (Fig. 4E) comprising anterior papilla bearing 3 unequal, naked setae and tapering posterior process. Maxilla 2-segmented (Fig. 4F), comprising elongate unarmed syncoxa and basis: basis bearing membranous subapical flabellum on anterior margin, and terminating in 2 subequal claw-like elements (calamus and canna). Calamus longer than canna, ornamented with strips of serrated membrane arranged obliquely around surface; canna ornamented with strips of serrated membrane. Maxilliped subchelate (Fig. 4G); proximal segment with smooth myxal surface; distal subchela with apical claw separated from proximal segmental part by incomplete suture; small seta present near concave margin. Sternal furca (Fig. 4H) with divergent, pointed tines.
First swimming leg pair (Fig. 5A) joined by slender intercoxal sclerite; sympod with inner and outer plumose setae derived from basis; endopod represented by unarmed process on posterior margin of basis. Exopod 2-segmented; directed laterally and forming main axis of leg; first segment about 2.5 times longer than wide and armed with small outer (anterior) spine and ornamented with row of setules along part of posterior margin; second segment about 2 times longer than wide, armed with 3 long plumose setae along posterior margin, and 4 distal elements along anterior and distal margins as follows: spines 1, 2 and 3 all of similar length, spines 2 and 3 lacking accessory process; seta 4 similar in length to spines 1 and shorter than segment.
Second leg (Fig. 5B) biramous, with flattened protopodal segments and 3-segmented rami. Coxae of leg pair joined by intercoxal sclerite bearing marginal membrane posteriorly. Coxa with plumose seta posteriorly and surface sensilla. Basis armed with outer naked spine, ornamented with surface sensilla and marginal membrane posteriorly, and with flap of membrane anteriorly, reflexed back over dorsal surface of segment. Exopodal segments 1 and 2 each with long, slightly-curved outer spine extending more-or-less parallel with main axis of ramus, each also armed with inner plumose seta; segment 1 also bearing flap of membrane anteriorly, reflexed back over dorsal surface of segment; segment 3 with 2 outer spines, proximal spine smaller than distal, both unornamented; apical spine with marginal membrane laterally and pinnules medially, and 5 inner plumose setae. Endopodal segment 1 armed with inner plumose seta, lacking ornamentation at outer distal angle; segments 2 and 3 both ornamented with patches of setules extending onto surface of segment; segment 2 armed with 2 inner plumose setae; segment 3 with 6 plumose setae.
Third leg pair (Fig. 5C) forming flattened plate closing posterior part of cephalothoracic sucker as typical for genus. Leg pair joined by plate-like, intercoxal sclerite (apron) ornamented with marginal membrane posteriorly. Protopodal part flattened, bearing inner plumose seta at junction with intercoxal plate, and outer plumose seta located dorsally near base of exopod; single sensillae located adjacent to inner coxal seta and adjacent to origin of endopod; ornamented with strips of membrane along posterior margin medial to endopod and along lateral margin anterior to exopod; Exopod 3-segmented; first segment armed with straight outer spine directed over ventral surface of ramus; second segment with small outer spine and inner plumose seta; third with 3 short outer spines and 4 inner plumose setae; outer margins of segments 2 and 3 ornamented with slender setules. Endopod 2-segmented; first segment forming long velum ornamented with fine setules along free margin and armed with inner plumose seta; compound distal segment with expanded outer margin and bearing 6 setal elements increasing in length from outermost to innermost.
Fourth leg (Fig. 5D) 4-segmented, comprising robust protopodal segment and 3-segmented exopod: protopodal segment armed with plumose seta distally; first exopodal segment armed with slender outer spine; second with 1 outer spine, third with 3 spines; all spines similar in length; each spine unilaterally fringed and with pecten at base expanded to form membranous strip along free margin of segment; first exopodal segment with minute sensilla on papilla on margin (Fig. 5D, insert) just proximal to strip-like pecten.
Fifth legs located laterally on genital complex (Fig. 4B); each fifth leg comprising anterior process bearing short plumose seta (representing outer protopodal seta) and inner exopodal process armed with 2 plumose setae.
Adult male (Fig. 6A) mean body length 2.69 mm (range 2.50 to 2.93 mm) including caudal rami (based on 10 specimens). Cephalothorax as in female. Genital complex (Fig. 6B) about 1.1 times longer than wide (0.50 x 0.47 mm); with slightly convex lateral margins bearing fifth legs about at mid-level. Abdomen 2-segmented with segments separated by deep constriction; first segment wider than long (0.12 mm x 0.21 mm), second segment about 2.3 times longer than first, and about 1.1 times longer than wide (0.27 x 0.24 mm); carrying paired caudal rami distally as in female.
Antennule, mandible, and maxilla as in female. Antenna modified (Fig. 6C); first segment elongate; second segment reflexed, bearing corrugated adhesion pads ventrally in distal part and anteriorly in proximal part; distal segment with well-developed apical claw plus strong accessory process equal in size to claw, segment bearing 2 setae proximally. Postantennal process (Fig. 6D) with better developed tine than in female; ornamented with 2 bisensillate papillae and with similar bisensillate papilla on adjacent ventral cephalic surface. Maxillule (Fig. 6E) with surface corrugations in middle part of posterior process. Maxilliped (Fig. 6F) with 2 processes on myxal margin of proximal segment (syncoxa); proximal process with bifid apex, distal process tooth-like; seta on subchela longer than in female, extending almost to tip of claw.
Legs 1 to 4 as in female. Leg 5 (Fig. 6B) represented by outer protopodal seta originating on somite surface and 2 setae on inner papilla representing exopod. Sixth leg represented by opercular plate closing off genital opening armed with 3 slender setae on outer distal corner.
Remarks: The new species belongs to a group of species recognized by Boxshall (2018) as the Caligus diaphanus-group. Members of this group are characterised by the following combination of shared character states: 3-segmented exopod on leg 4 armed with I, I, III spines and typically ornamented with a linear strip of membrane (the modified pecten) associated with the base of each spine; three plumose setae present on posterior margin of distal exopodal segment of leg 1; spines 2 and 3 on distal exopodal segment of leg 1 lacking accessory processes; leg 2 with an ornamentation of fine setules extending from the margin over onto surface of both the second and third endopodal segments, and with the outer spines of the first and second exopodal segments aligned close to the longitudinal axis of the ramus; the female antenna lacking a posterior process on the proximal segment; and the tine on the post-antennal process vestigial or weakly developed. The males are typically characterised by the presence of a well developed accessory process on the distal claw of the antenna.
Boxshall (2018) established this group to include: C. diaphanus von Nordmann, 1832, C. cybii Bassett-Smith, 1898, C. fajerae Morales-Serna, Oceguera-Figueroa & Tang, 2017, C. kanagurta Pillai, 1961, C. kapuhili Lewis, 1967, C. laticaudus Shiino, 1960, C. macrurus Heller, 1865, C. stromatei Krøyer, 1863 (syn. C. multispinosus Shen, 1957), C. pagelli Delamare Deboutteville & Nuñes-Ruivo, 1958, C. pelamydis Krøyer, 1863, C. platytarsis Bassett-Smith, 1898, C. robustus Bassett-Smith, 1898, C. rotundigenitalis Yü, 1933, C. tanago Yamaguti, 1939, and C. tenuis (van Beneden, 1852). Boxshall & Bernot (submitted) noted that C. pagri Capart, 1941 also belongs in the C. diaphanus-group. Here we place the new species C. auriolus n. sp. in this group. These species can be distinguished with the aid of the following key to adult females:
-
1.
Abdomen hyper-elongate, longer than cephalothorax and genital complex combined……2
Abdomen shorter than cephalothorax and genital complex combined…………………………3
-
2.
Sternal furca present; caudal rami 4.0 to 5.0 times longer than wide...……… C. macrurus
Sternal furca absent; caudal rami 2.0 to 2.4 times longer than wide……………… C. tenuis
-
3.
Maxilliped of female with large myxal process…………………………………….………….4
Maxilliped of female with smooth myxal margin …………………………………………..…7
-
4.
Abdomen less than half length of genital complex……………….………..……..C. kapuhili
Abdomen more than 60 to 65% of length of genital complex ………….……………………5
-
5.
Myxal region of maxilliped with large proximal process plus adjacent spiniform process on margin……………………………C. robustus
Myxal region of maxilliped with large tapering process only on margin……………6
-
6.
Abdomen longer than genital complex………………………………… C. diaphanus
Abdomen shorter than genital complex………………………………………….C. laticaudus
-
7.
Pectens on leg 4 exopod modified as hirsute digitiform processes….……….C. kanagurta
Pectens modified as linear strips of membrane along margin of segments………..……...8
-
8.
All exopodal spines on leg 4 about equal in length and directed away from ramus……....9
Spine on first exopodal segment of leg 4 orientated in parallel with outer margin of second segment………………………………13
-
9.
Outer margin spines on exopodal segments 1 and 2, plus proximal 2 spines on segment
3 of leg 4 swollen and ornamented with short hairs over surface…..………10
These spines on leg 4 slender and tapering towards tip, ornamented with strips of membrane and/or rows of setules……………………………11
-
10.
Sternal furca with extremely flattened, spatulate tines that are wider than long; post-antennal process without tine…………………………C. platytarsis
Sternal furca with tines longer than wide; post-antennal process with small tine…C. tanago
-
11.
Abdomen less than 2 times longer than wide; protopodal segment of leg 4 swollen, about 2.0 times longer than wide…………………….C. auriolus n. sp.
Abdomen about 3 times longer than wide; protopodal segment of leg 4 slender, about 2.5 times longer than wide………………………………12
-
12.
Outer spine on first exopodal segment of leg 3 not reaching articulation between second and third segments; tine of vestigial post-antennal process shorter than base…………………………………………………………C. pelamydis
Outer spine on first exopodal segment of leg 3 reaching beyond articulation between second and third segments; tine of vestigial post-antennal process longer than base………………………………………………….…..C. stromatei
-
13.
Genital complex wider than long……………………………………………14
Genital complex 1.25 times longer than wide……………………………C. fajerae
-
14.
Abdomen markedly longer than genital complex, distinctly wider anteriorly and tapering evenly towards posterior ……………………C. cybii
Abdomen shorter than or as long as genital complex…………………………………….15
-
15.
First abdominal somite about 4 times longer than anal somite…………............C. pagelli
First abdominal somite at most 2 times longer than anal somite……………………….16
-
16.
Lateral margins of genital complex strongly rounded; outer spine on first exopodal segment of leg 3 reaching beyond articulation between second and third segments …………………………………C. rotundigenitalis
Lateral margins of genital complex linear to slightly convex; outer spine on first exopodal segment of leg 3 not reaching articulation between second and third segments……………………………………………………………C. pagri
Within the C. diaphanus-group, the new species appears to be closely related to C. pelamydis and C stromatei. All three species share the possession of: a smooth myxal margin on the female maxilliped, pectens modified as linear strips on leg 4, and with all exopodal spines on leg 4 tapering to a pointed tip and directed outwards at an angle to the ramus. The female of C. auriolus n. sp. differs from C. pelamydis in its shorter abdomen, which is 1.5 times longer than wide compared to about 3.8 times longer in the latter species. Similarly, the male of C. auriolus n. sp. differs in having the anal somite about 1.1 times longer than wide compared to about 1.5 times in C. pelamydis. The form of the myxal process on the male maxilliped also differs between these two species. In male C. pelamydis the myxal process is a small, distally-bifid lobe (see Cressey & Cressey, 1980: fig. 66F) whereas in male C. auriolus n. sp. the myxal margin carries a simple distal process and a bifid proximal process.
Comparison of C. auriolus n. sp. with the description of C. stromatei in Ho & Lin (2004) (as C. multispinosus) reveals numerous close similarities even in fine details, such as the presence of a minute sensilla on the margin of the first exopodal segment of leg 4 in the female (Fig. 5D) just proximal to the linear, strip-like pecten and the unusually long seta on the male maxilliped (Fig. 6F). However, these species can be distinguished by: the proportions of the abdomen which is about 2.9 times longer than wide in C. stromatei but only 1.5 times longer in the new species; the shape of the sternal furca which has tapering divergent tines in the new species compared with rounded, more or less parallel tines in C. stromatei; and by the length of the spine on exopod segment 1 of leg 3, which extends beyond the articulation between segments 2 and 3 in C. stromatei but is short and does not reach this articulation in the new species. In the male of C. stromatei the maxilliped bears a tiny conical myxal process (Ho & Lin, 2004: Fig. 109E, as C. multispinosus) where in the male of C. auriolus n. sp. the myxal process comprises a simple distal process and a bifid proximal process. These differences are sufficient to differentiate between these two species.
Discussion
Adult males and chalimus stages but only pre-metamorphic adult females of C. dussumieri were found on L. johnii. It is possible that the absence of metamorphosed females might simply reflect a change in microhabitat on the host but an alternative explanation is that a host switching event occurs during the life cycle, with the mated adult female switching to a Dussumieria species as a final host. Two host life cycles are uncommon in parasitic copepods, although species of some genera (but not all, see Ismail et al., 2013) within the family Pennellidae are confirmed as utilizing two hosts, including Lernaeocera de Blainville, 1822 which uses different fishes as both first and second hosts (Sproston, 1942), Cardiodectes Wilson, 1917, one species of which uses pelagic molluscs as the first host and fish as the second (Perkins, 1983), and Pennella Oken, 1815 which uses squid as first host and a marine vertebrate such as a whale or a fish, as second host (e.g. Pascual et al., 2001). However, life cycles involving two hosts have also been suggested for caligid copepods. Hayward et al. (2011), for example, found the developing chalimus stages of Caligus chiastos Ho & Lin, 2003 on Thamnacornus degeni (Regen) outside of sea cages used to farm southern bluefin tuna (Thunnus maccoyii (Castelnau)), while adults only were present on the farmed tuna within the cages.
Cressey & Cressey (1980) considered that species of Scomberomorus Lacépède, 1801 served as hosts only for immature stages of Caligus biseriodentatus and that adults appear to be found on a different host from the immature stages. They recognized the species originally described as C. auxisi by Pillai (1963) as the adult of C. biseriodentatus and the only host known to harbour an adult was Auxis thazard (Lacépède). Cressey & Cressey (1980) speculated that the adult may prefer a non-scombrid host but the recent discovery of two more adult females of C. biseriodentatus on A. thazard from Moreton Bay led Boxshall (2018) to infer that this scombrid may well be the preferred host of the adult.
It seems likely that C. dussumieri might be another example of a Caligus utilizing two hosts in its life cycle, with development in Australian waters taking place on Lutjanus johnii up to and including mating. After mating the fertilized adult female then switches to the second host, probably a clupeiform fish (given the known hosts of this species), where it completes its post-mating metamorphosis and commences egg string production. The new synonymy recognized here suggests that in Indian waters, C. dussumieri may use Lutjanus rivulatus as the first host before switching to a second host.
References
Boxaspen, K. K., Karlsen, Ø., Svåsand, T., & Asplin, L. (2022). Impacts of sea lice. In: Treasurer, J., Bricknell, I., & Bron, J. (Eds), Sea Lice Biology and Control. 5M Books Ltd, pp 531–547.
Boxshall, G. A. (1990a). The skeletomusculature of siphonostomatoid copepods, with an analysis of adaptive radiation in structure of the oral cone. Philosophical Transactions of the Royal Society of London Series B, 328, 167–212.
Boxshall, G. A. (1990b). Precopulatory mate guarding in copepods. Bijdragen tot de Dierkunde, 60, 209–213.
Boxshall, G. A. (2018). The sea lice (Copepoda: Caligidae) of Moreton Bay (Queensland, Australia), with descriptions of thirteen new species. Zootaxa, 4398, 1–172.
Boxshall G. A., & Bernot, J. P. (submitted). Resolving taxonomic and nomenclatural problems in the genus Caligus O.F. Müller, 1785 (Copepoda: Caligidae). Zootaxa,
Boxshall, G. A., & Montú, M. (1997). Copepods Parasitic on Brazilian Coastal Fishes: A Handbook. Nauplius, 5, 1–225, 89 pls.
Boxshall, G. A., & Özak, A. A. (2022). Introduction to sea lice morphology and biology. In: Treasurer, J., Bricknell, I. & Bron, J. (Eds) Sea Lice Biology and Control. 5M Books Ltd, pp 13–49.
Boxshall, G. A., Bernot, J. P., Barton, D. P., Diggles, B. K., Yong, R. Q-Y., Atkinson-Coyle, T., & Hutson, K. S. (2020). Parasitic copepods of the family Lernanthropidae Kabata, 1979 (Copepoda: Siphonostomatoida) recorded from Australian fishes, with descriptions of seven new species. Zootaxa, 4736, 1–103
Cressey, R.F. & Cressey, H.B. (1980). Parasitic copepods of mackerel- and tuna-like fishes (Scombridae) of the world. Smithsonian Contributions to Zoology, 311, 1–86.
Diggles, B. K., Barnes, L., Landos, M., Dennis, M. M., & O'Carroll, J. P. J. (2021). Sea lice Lepeophtheirus spinifer, Tuxophorus sp. and Caligus sp. infections on wild-caught queenfish Scomberoides commersonnianus from northern Australia. Diseases of Aquatic Organisms, 143, 37–50.
Dojiri, M., & Ho, J.-s. (2013). Systematics of the Caligidae, copepods parasitic on marine fishes. Crustaceana Monographs 18: Brill, Leiden, Boston. 448 pp.
Froese, R., & Pauly, D. (Editors) (2022). FishBase. World Wide Web electronic publication. www.fishbase.org, accessed 06 October 2022.
Gnanamuthu, C. P. (1950). Parapetalus caudatus n. sp., a copepod parasitic on Dussumieria acuta from Madras. Proceedings of the Indian Academy of Sciences (B), 31(2), 125–133, figs. 1-5.
Hayward, C. J., Svane, I., Lachimpadi, S. K., Itoh, N., Bott, N. J., & Novak, B. F. (2011). Sea lice infections of wild fishes near ranched southern Bluefin tuna (Thunnus maccoyi) in South Australia. Aquaculture, 320, 178–182.
Ho, J.-s. & Lin, C.-L. (2004) Sea Lice of Taiwan (Copepoda: Siphonostomatoida: Caligidae). The Sueichan Press, Keelung, 388 pp.
Huys, R., & Boxshall, G. A. (1991). Copepod Evolution. The Ray Society, London. 468 pp.
Ismail, N., Ohtsuka, S., Venmathi Maran, B. A., Tasumi, S., Zaleha, K., & Yamashita, H. (2013). Complete life cycle of a pennellid Peniculus minuticaudae Shiino, 1956 (Copepoda: Siphonostomatoida) infecting cultured threadsail filefish, Stephanolepis cirrhifer. Parasite, 20, 42, doi: https://doi.org/10.1051/parasite/2013041.
Izawa, K. (1995). A new fish parasite (Copepoda: Siphonostomatoida: Caligidae) from the Timor Sea, Australia. Beagle, Occasional Papers of the Northern Territory Museum of Arts and Sciences, Darwin, 12, 185–192.
Johnson, S. C., Treasurer, J. W., Bravo, S., Nagasawa, K., & Kabata, Z. (2004). A review of the impact of parasitic copepods on marine aquaculture. Zoological Studies, 43, 229–243.
Pascual, S., Gonzalez, A. E., Gestal, C., Abollo, E., & Guerra, A. (2001). Epidemiology of Pennella sp. (Crustacea: Copepoda), in exploited Illex coindetii stock in the NE Atlantic. Scientia Marina, 65, 307–312.
Perkins, P. S. (1983). The life history of Cardiodectes medusaeus (Wilson), a copepod parasite of lanternfishes (Myctophidae). Journal of Crustacean Biology, 3, 70–87.
Pilla, S., Vankara, A. P., & Chikkam, V. (2012). Copepod parasites of snappers Lutjanus spp. (Pisces, Lutjanidae) with description of a new caligid copepod Caligus rivulatus sp. nov. (Copepoda, Caligidae) from Visakhapatnam coast, India. Cibtech Journal of Zoology, 1(2), 16–24.
Pillai, N. K. (1962). A revision of the genera Parapetalus Steenstrup & Lütken and Pseudopetalus nov. Crustaceana, 3, 285–303, figs. 1–7.
Pillai, N. K. (1963). Copepods parasitic on South Indian fishes: Family Caligidae. Journal of the Marine Biological Association of India, 5, 68–96, figs. 1–16.
Pillai, N. K. (1968). Additions to the copepod parasites of South Indian fishes. Parasitology, 58, 9–36.
Pillai, N. K. (1985). Fauna of India. Parasitic copepods of marine fishes. Zoological Survey of India, Calcutta, 900 pp.
Rangnekar, M. P. (1957). Caligus dasyaticus sp. nov. and Caligus dussumieri sp. nov. (Copepoda) parasitic on Bombay fishes. Journal of the University of Bombay, 25, 16–22.
Redkar, M. V., Rangnekar P. G., & Murti, N. N. (1949). Four new species of parasitic copepods from the marine fishes of Bombay. Journal of the University of Bombay (B), 18(3), 36–50, pls. 1–4.
Shen, C. J. (1957). Parasitic copepods from fishes of China. Part II. Caligoida, Caligidae (1). Acta Zoologica Sinica, 9, 351–378, pls 1–11.
Sproston, N. G. (1942). The developmental stages of Lernaeocera branchialis (Linn.) Journal of the Marine Biological Association of the United Kingdom, 25, 441–466.
Acknowledgements
The authors thank the staff of the Northern Territory Department of Industry, Tourism and Trade, the Department of Fisheries Western Australia, and the Queensland Department of Agriculture and Fisheries Long Term Monitoring Program, the Indigenous Marine Rangers from Borroloola, Maningrida, Wadeye, Larrakia, Wunambal Gaambera, Dambimangari and Bardi-Jawi as well as various recreational and commercial fishers, for the collection of fish.
Funding
This study was supported by the Australian Fisheries Research and Development Corporation (Project Number FRDC 2013/017).
Author information
Authors and Affiliations
Contributions
DPB collected the material, GAB prepared figures 1-6, and both authors wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
The fish collection was conducted under Charles Darwin University Animal Ethics Approval A13014.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boxshall, G., Barton, D.P. Caligid sea lice (Copepoda: Caligidae) from golden snapper Lutjanus johnii (Bloch) in Australian waters, with the recognition of Sinocaligus Shen, 1957 as a junior synonym of Caligus Müller, 1785. Syst Parasitol 100, 487–504 (2023). https://doi.org/10.1007/s11230-023-10099-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-023-10099-z