Abstract
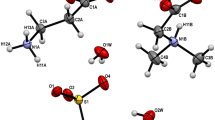
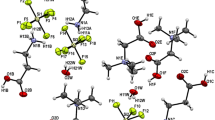
Recently, we have discovered a new class of amino acids salts containing different amino acids. In the present paper, we report crystal structures of three isostructural salts: l-argininium( +) sarcosine chloride (I), l-argininium( +) sarcosine bromide (II), and l-argininium( +) sarcosine iodide (III), formed by slow evaporation at room temperature from aqueous solutions containing stoichiometric ratios of components. The compounds crystallize in the polar space group P21 with two formula units in the asymmetric unit. The structures are stabilized due to N–H···O and N–H···X (X = Cl, Br, I) hydrogen bonds. Infrared spectra of all three crystals are shown and discussed.




Similar content being viewed by others
Availability of data and materials
Further crystallographic data have been deposited with the Cambridge Crystallographic Data Centre and can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336,033), citing the title of this paper and the CCDC nos. 2162926 (I), 2,162,925 (II), 2,162,923 (III), and 2,162,924 ((l-ArgH)Br).
Code availability
CCDC nos. 2162923, 2,162,924, 2,162,925, 2,162,926.
References
Fleck M, Petrosyan AM (2014) Salts of amino acids: crystallization, structure and properties. Springer, Dordrecht
Petrosyan AM, Giester G, Tonoyan GS, Ghazaryan VV, Fleck M (2019) A new class of amino acids salts. Abstracts of the 3rd German Polish Conference on Crystal Growth, March 17–21, Poznań, Poland, 57
Petrosyan AM, Giester G, Tonoyan GS, Ghazaryan VV, Fleck M (2021) Salts containing different amino acids: three types salts of l-arginine containing glycine, dimethylglycine or β-alanine. J Mol Struct 1228:129717
Tonoyan GS, Giester G, Petrosyan AM (2022) Salts containing different amino acids: Salts with β-alaninium l-proline dimeric cation. J Mol Struct 1252:132171
Mazumdar SK, Srinivasan R (1964) X-ray analysis of l-arginine hydrohalides, Curr Sci 19:573–575
Mazumdar SK, Srinivasan R (1966) The crystal structure of l-arginine monohydrobromide monohydrate. Z Kristallogr 123:186–205
Jean D, Jensen LH, Mazumdar SK, Srinivasan R, Ramachandran GN (1970) Refinement of the structure of arginine hydrochloride monohydrate. Acta Crystallogr B 26:1662–1671
Sridhar B, Srinivasan N, Bjoern D, Rajram RK (2002) A triclinic polymorph of l-argininium chloride. Acta Crystallogr E58:747–749
Seely Jr O (1966) The crystal structure analysis of l-arginine dihydriodide and l-tyrosine hydriodide. PhD thesis, University of Illinois, Urbana, Illinois, 183
Monaco SB, Davis LE, Velsko SP, Wang FT, Eimerl D, Zalkin A (1987) Synthesis and characterization of chemical analogs of l-arginine phosphate. J Cryst Growth 85:252–255
Petrosyan AM, Sukiasyan RP, Karapetyan HA, Terzyan SS, Feigelson RS (2000) Growth and investigation of new nonlinear optical crystals of LAP family. J Cryst Growth 213:103–111
Petrosyan AM, Feigelson RS, Van Stryland EW, Sukiasyan RP, Karapetyan HA (2002) New class of nonlinear optical crystals among arginine salts. Proc SPIE 4751:217–222
Petrosyan AM, Karapetyan HA, Bush AA, Sukiasyan RP (2004) Crystal structure and characterization of l-arginine dichloride monohydrate and l-arginine dibromide monohydrate. Mater Chem Phys 84:79–86
John JS, Kumar MS, Joy LK, Sajan D, Vinitha G, Vijayan N, John NL (2020) Crystal growth, dielectric studies, charge transfer and ionic hydrogenbonding interactions of l-arginine hydrobromide monohydrate single crystal: a novel third order nonlinear optical material for optoelectronic applications. Opt Laser Technol 125:106043
Mukerji S, Kar T (1998) Structural, thermal and spectroscopic investigation of nonlinear optical crystal l-arginine hydrobromide monohydrate. Mater Res Bull 33:619–626
Schmid H (1968) Addition compounds of amino acids and hydrofluoric acid or soluble fluorides, and method of preparing the same. US Patent # 3,413,326
Ramos Silva M, Paixão JA, Matos Beja A, Alte da Veiga L (2000) Crystal structure of the nonlinear optical compound l-arginine fluoride. J Chem Cryst 30:411–414
Ramos Silva M, Paixão JA, Matos Beja A, Alte da Veiga L (2000) Very short F-H···F hydrogen bond in l-argininum fluoride hydrogen fluoride. Acta Crystallogr C 56:104–106
Bhattacharyya SC, Saha NN (1978) Crystal and molecular structure of sarcosine hydrochloride. J Cryst Mol Struct 8:105–113
Bhattacharyya SC, Saha NN (1978) Crystal and molecular structure of disarcosine hydrobromide. J Cryst Mol Struct 8:209–215
Ghazaryan VV, Fleck M, Petrosyan AM (2012) Sarcosine sarcosinium chloride and sarcosine sarcosinium bromide. J Mol Struct 1020:160–166
Ghazaryan VV, Fleck M, Petrosyan AM (2013) Iodides of sarcosine. J Mol Struct 1032:35–40
Petrosyan AM, Ghazaryan VV, Giester G, Fleck M, Tylczyński Z, Wiesner W (2018) Halogenides of dimethylglycine in comparison with respective salts of glycine, sarcosine and betaine. J Mol Struct 1158:106–121
Bruker (2020) Apex3 suite – Bruker AXS. Karlsruhe, Germany
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Hübschle CB, Sheldrick GM, Dittrich B (2021) ShelXle: a Qt graphical user interface for SHELXL. J Appl Cryst 44:1281–1284
Parsons S, Flack HD, Wagner T (2013) Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr B 69:249–259
Görbitz CH (2015) Crystal structures of amino acids: from bond lengths in glycine to metal complexes and high-pressure polymorphs. Cryst Rev 21:160–212
Salunke DM, Vijayan M (1981) Specific interactions involving guanidyl group observed in crystal structures. Int J Pept Protein Res 18:348–351
Saraswathi NT, Siddhartha R, Vijayan M (2003) X-ray studies on crystalline complexes involving amino acids and peptides. XLI. Commonalities in aggregation and conformation revealed by the crystal structures of the pimelic acid complexes of l-arginine and dl-lysine. Acta Crystallogr B 59:641–646
Petrosyan AM, Ghazaryan VV, Tonoyan GS, Aghajanova EM, Giester G, Fleck M (2020) Salts containing different amino acids and iodide anion as regulators of thyroxine synthesis. Armenian Patent # 656 Y
Gómez-Zavglia A, Fausto R (2003) Low-temperature solid-state FTIR study of glycine, sarcosine and N, N-dimethylglycine: observation of neutral forms of α-amino acids in the solid state. Phys Chem Chem Phys 5:3154–3161
Funding
This work was supported by the RA MES Committee of Science, in the frames of the research project № 18 T-1D033.
Author information
Authors and Affiliations
Contributions
Aram M. Petrosyan: conceptualization, writing — original draft, review and editing. Gerald Giester: investigation, formal analysis, writing — review and editing. Gayane S. Tonoyan: investigation, visualization, writing — review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tonoyan, G.S., Giester, G. & Petrosyan, A.M. Salts containing different amino acids: l-argininium ( +) sarcosine halogenides. Struct Chem 35, 943–952 (2024). https://doi.org/10.1007/s11224-023-02246-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02246-5