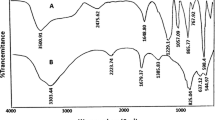
Abstract
The condensation of ethyl cyanoacetate and salicylaldehyde would afford either coumarin-3-carboxylate ester or 3-cyancoumarin as the final product. Herein, comparative experiments and density functional theory (DFT) calculations were investigated to pursue a deeper understanding of the critical factor in this reaction. Experimental results indicated that condensation is accomplished through a cascade process, including the Knoevenagel procedure followed by selective cyclization of the phenolic hydroxyl group to the cyano or ester carbonyl group within the intermediate. Product distribution correlates well with the acid–base properties of the catalyst and the ability of the catalyst for forming complex by hydrogen bonds. Also, an efficient and facile approach was developed for the preparation of coumarin-3-carboxylate ester as a major product from the reaction of ethyl cyanoacetate with salicylaldehyde. Low-transition-temperature mixture (LTTM) formed from L-proline and oxalic acid was proved as an inexpensive, easily available, and efficient promoter which not only affords the products in high yields but also avoids the use of hazardous solvent and tedious isolation procedures.








Similar content being viewed by others
Availability of data and materials
The data and materials are available with requirements.
References
Grover J, Jachak SM (2015) RSC Adv 5:38892–38905
Cao D, Liu Z, Verwilst P, Koo S, Jangjili P, Kim JS, Lin W (2019) Chem Rev 119:10403–10519
Francisco CS, Francisco CS, Constantino AF, Neto ÁC, Lacerda V Jr (2019) Curr Org Chem 23:2722–2750
Lončarić M, Gašo-Sokač D, Jokić S, Molnar M (2020) Biomolecules 10:151
Singh J, Sharma A (2021) Adv Synth Catal 363:3411–3438
Cerqueira NMFSA, Rodrigues LM, Oliveira-Campos AMF, Carvalho LHM, Coelho PJ, Dubest R, Aubard J, Samat A, Guglielmetti R (2003) Helv Chim Acta 86:3244–3253
Ni S, Zhou J, Mei H, Han J (2018) Tetrahedron Lett 59:1309–1316
Khan D, Mukhtar S, Alsharif MA, Alahmdi MI, Ahmed N (2017) Tetrahedron Lett 58:3183–3187
Sairam M, Saidachary G, Raju BC (2015) Tetrahedron Lett 56:1338–1343
Gao SQ, Xiao D, Yang Y, Wei XY, Sun S, Lang J, Lü CW (2016) Heterocycles 92:1698–1705
Pan WY, Xiao YM, Xiong HQ, Lü CW (2016) Res Chem Intermediat 42:7057–7063
Xiao YD, Liu A, Gao JL, Zou YZ, Lü CW, An Y (2020) Dyes Pigments 179:108415
Gao JL, Liu A, Li MH, Wang YY, Xiao YD, Lü CW, An Y (2021) Res Chem Intermediat 47:3179–3187
Bmfola G, Fringuelli F, Piermatti O, Pizzo F (1996) Heterocycles 43:1257–1266
Chen J, Zhang Y (2001) J Chem Research(s) 394–395
He X, Yan Z, Hu X, Zuo Y, Jiang C, Jin L, Shang Y (2014) Synth Commun 44:1507–1514
Jing Y, Meng J, Liu Y, Wan J-P (2015) Chin J Chem 33:1194–1198
Shockravi A, Shargi H, Valizadeh H, Heravic MM (2002) Phosphorus Sulfur Silicon 177:2555–2559
Valizadeh H, Mamaghani M, Badrian A (2005) Synth Commun 35:785–790
Valizadeh H, Shockravi A, Gholipur H (2007) J Heterocyclic Chem 44:867–870
Augustine JK, Bombrun A, Ramappa B, Boodappa C (2012) Tetrahedron Lett 53:4422–4425
Phadtare SB, Shankarling GS (2012) Environ Chem Lett 10:363–368
Devulapally S, Syed T, Pramod KD (2014) Lett Org Chem 11:556–563
Kiyani H, Daroonkala MD (2015) Bull Chem Soc Ethiop 29:449–456
Keshavarzipour F, Tavakol H (2016) J Iran Chem Soc 13:149–153
da Silveira Pinto LS, de Souza MV (2017) Synthesis 49:2677–2682
Volmajer J, Toplak R, Leban I, Le MAM (2005) Tetrahedron 61:7012–7021
He X, Shang Y, Zhou Y, Yu Z, Han G, Jin W, Chen J (2015) Tetrahedron 71:863–868
Francisco M, van den Bruinhorst A, Kroon MC (2012) Green Chem 14:2153–2157
Francisco M, van den Bruinhorst A, Kroon MC (2013) Angew Chem Int Ed 52:3074–3085
Płotka-Wasylka J, de la Guardia M, Andruch V, Vilková M (2020) Microchem J 159:105539
Marenich AV, Cramer CJ, Truhlar DG (2009) J Phys Chem B 113:6378–6396
Ho J, Klamt A, Coote ML (2010) J Phys Chem A 114:13442–13444
Acknowledgements
We are grateful for the Scientific Research Fund of Liaoning Provincial Education Department (No. LJKQZ2021092, LJKMZ20221433).
Funding
Scientific Research Fund of Liaoning Provincial Education Department (No. LJKQZ2021092, LJKMZ20221433).
Author information
Authors and Affiliations
Contributions
Chengwei Lü contributed to the study conception and design and reviewed the manuscript. Material preparation and data collection were performed by Si-Han Wei and Lu-Yang Qin. Analysis was conducted by Yu Luo and Si-Yu Chen. The first draft of the manuscript was written by Mo-Han Yu. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, SH., Luo, Y., Chen, SY. et al. Study insights into the different cyclization mechanisms of ethyl cyanoacetate with salicylaldehyde and efficient synthesis of coumarin-3-carboxylate ester. Struct Chem 35, 341–348 (2024). https://doi.org/10.1007/s11224-023-02194-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02194-0