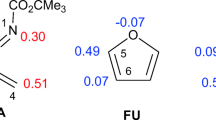
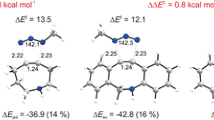
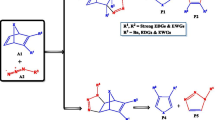
Abstract
The molecular mechanism of the cycloaddition reactions of 2H-azirine with 1-methoxybutadiene and cyclohexadiene has been studied at the M06-2X/cc-pVDZ level of theory. Analysis of the energy results indicated that the meta/endo pathways are the most stable paths for all studied reactions. The presence of Lewis acid in these reactions decreases the activation energies markedly and increases the diastereoselectivities. Moreover, the nucleophilicity and electrophilicity of the reagents and selectivity of the reactions were analyzed by DFT reactivity indices. Analysis of electron localization function (ELF) indicated that the general processes of electronic changes for the meta/endo pathways of the cycloaddition reactions of 2H-azirine 1 and 1-Zn with 1-methoxy-1,3-butadiene 2 are similar, and both reactions take place via the one-step two-stage mechanism. However, in the presence of Lewis acid (LA) catalyst, the fragments are more separated, and the reaction proceeds through an earlier transition state (TS). The non-covalent interaction (NCI) topological analysis at the transition states reveals that only one strong, attractive interaction between the reaction sites is responsible for the highly asynchronous process in the presence of Lewis acids.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Huang C-Y, Doyle AG (2014) The chemistry of transition metals with three-membered ring heterocycles. ACS Publ 114:8153–8198. https://doi.org/10.1021/cr500036t
Zhou H, Shen MH, Xu HD (2016) Evolution of the Aza-Diels-Alder reaction of 2 H -Azirines. Synlett 27:2171–2177. https://doi.org/10.1055/s-0035-1562493
Räber JL, Stoykova SA, Strässler C (2013) New 2H-azirin-3-amines as synthons for sulfur-heterocyclic α -amino acids new 2H-azirin-3-amines as synthons for sulfur-heterocyclic α-amino acids. Taylor Fr 188:441–445. https://doi.org/10.1080/10426507.2012.729114
H Hemetsberger DK Monthly LastName1972 undefined, (1972) Synthese von 1-aryl-2-azido-2-alken-1-onen Monatshefte für Chemie/Chemical 103:205–209
Alves MJ, Durães MM, Fortes AG (2004) Diels-Alder cycloaddition of 2-azadienes to methyl 2-(2,6-dichlorophenyl)- 2H-azirine-3-carboxylate in the synthesis of methyl 4-oxo-1,3-diazabicyclo[4.1. 0]heptane-6-carboxylates. Tetrahedron 60:6541–6553. https://doi.org/10.1016/j.tet.2004.06.004
Alves MJ, Fortes AG, Costa FT (2006) Diels-Alder cycloaddition of electrophilic 2H-azirines with 3-(3-(tert-butyldimethylsilyloxy)buta-1,3-dienyl)oxazolidin-2-ones. Treatment of the cycloadducts under acidic conditions. Tetrahedron 62:3095–3102. https://doi.org/10.1016/j.tet.2006.01.035
Dubinina G, Yoshida W, Chain WJ (2020) On the preparation of azepinones. Tetrahedron Lett 51:5325–5327
Dubinina G, Chain WJ (2011) Reactions of azepinones with electrophiles. Tetrahedron Lett 52:939–942
Alves MJ, Fortes AG, Costa FT, Duarte VCM (2007) Formation of pyridin-4(1H)-one versus 1H-azepin-4(7H)-one by treatment of 4-tert-butyldimethylsilyloxy-2-amino-1-aza-bicyclo[4.1.0]hept-3-enes with tetrabutylammonium fluoride. Tetrahedron 63:11167–11173. https://doi.org/10.1016/j.tet.2007.08.007
Khlebnikov AF, Novikov MS, Rostovskii NV (2019) Advances in 2H-azirine chemistry: a seven-year update. Tetrahedron 75:2555–2624. https://doi.org/10.1016/j.tet.2019.03.040
Khlebnikov AF, Novikov MS (2013) Recent advances in 2H-azirine chemistry. Tetrahedron 69:3363–3401. https://doi.org/10.1016/j.tet.2013.02.020
Alves MJ, Costa C, Durães MM (2009) Diastereoselective Diels-Alder cycloaddition of [(1R)-10-(N, N-diethylsulfamoyl)isobornyl] 2H-azirine to nucleophilic 1,4-disubstituted 1,3-dienes. Tetrahedron Asymmetry 20:1378–1382. https://doi.org/10.1016/j.tetasy.2009.05.035
Timén ÅS, Fischer A, Somfai P (2003) Stereoselective aza-Diels–Alder reactions with 2H-azirines as dienophiles furnishing highly functionalized tetrahydropyridines. Chem Commun 3:1150–1151. https://doi.org/10.1039/b300849e
Timén ÅS, Somfai P (2003) Investigation of Lewis acid-catalyzed asymmetric aza-Diels-Alder reactions of 2H-Azirines. J Org Chem 68:9958–9963. https://doi.org/10.1021/jo0352326
Savin A, Becke AD, Flad J et al (1991) A new look at electron localization. Angew Chemie Int Ed English 30:409–412. https://doi.org/10.1002/anie.199104091
Savin A, Nesper R, Wengert S, Fässler TF (1997) ELF: the electron localization function. Angew Chemie (International Ed English) 36:1808–1832. https://doi.org/10.1002/anie.199718081
Savin A, Silvi B, Colonna F (1996) Topological analysis of the electron localization function applied to delocalized bonds. Can J Chem 74:1088–1096. https://doi.org/10.1139/v96-122
Noury S, Colonna F, Savin A, Silvi B (1998) Analysis of the delocalization in the topological theory of chemical bond. J Mol Struct 450:59–68. https://doi.org/10.1016/S0022-2860(98)00413-X
Johnson ER, Keinan S, Mori-Sánchez P et al (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. https://doi.org/10.1021/ja100936w
Lan Y, Zou L, Cao Y, Houk KN (2011) Computational methods to calculate accurate activation and reaction energies of 1,3-dipolar cycloadditions of 24 1,3-dipoles. J Phys Chem A 115:13906–13920. https://doi.org/10.1021/jp207563h
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094. https://doi.org/10.1021/cr00031a013
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041. https://doi.org/10.1063/1.474659
Hehre WJ, Radom L, PvR S, Pople JA (1986) Ab initio molecular orbital theory. John Wiley, NewYork
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746. https://doi.org/10.1063/1.449486
Domingo LR (2014) A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32428. https://doi.org/10.1039/c4ra04280h
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) A new model for C-C bond formation processes derived from the molecular electron density theory in the study of the mechanism of [3+2] cycloaddition reactions of carbenoid nitrile ylides with electron-deficient ethylenes. Tetrahedron 72:1524–1532. https://doi.org/10.1016/j.tet.2016.01.061
Blanchard P, Brüning E (2015) Density functional theory of atoms and molecules. Prog Math Phys 69:563–573
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study J Org Chem 73:4615–4624. https://doi.org/10.1021/jo800572a
Domingo LR, Pérez P (2011) The nucleophilicity N index in organic chemistry. Org Biomol Chem 9:7168–7175. https://doi.org/10.1039/c1ob05856h
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494. https://doi.org/10.1039/c2ra22886f
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery, JA Jr, Start-mann RE, Burant JC, Daprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochtersk Ji, Petersson GA, Ayala Y, Ui QC, Morokuma K, Malick DK, Rubuck AD, Raghavachari K, Foresman JB, Cioslowski J, Oritz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Comperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challa-combe M, Gill MW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople J.A, Gaussian 09 revision A.02, (2009), Gaussian, Inc, Wallingford CT
Noury S, Krokidis X, Fuster F, Silvi B (1999) Computational tools for the electron localization function topological analysis. Comput Chem 23:597–604. https://doi.org/10.1016/S0097-8485(99)00039-X
Contreras-García J, Johnson ER, Keinan S et al (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632. https://doi.org/10.1021/ct100641a
Parr RG, Yang W (1995) Density-functional theory of the electronic structure of molecules. Annu Rev Phys Chem 46:701–728. https://doi.org/10.1146/annurev.pc.46.100195.003413
Ess DH, Jones GO, Houk KN (2006) Conceptual, qualitative, and quantitative theories of 1,3-dipolar and Diels-Alder cycloadditions used in synthesis. Adv Synth Catal 348:2337–2361
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423. https://doi.org/10.1016/S0040-4020(02)00410-6
Jaramillo P, Domingo LR, Chamorro E, Pérez P (2008) A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J Mol Struct THEOCHEM 865:68–72. https://doi.org/10.1016/j.theochem.2008.06.022
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140. https://doi.org/10.1103/PhysRev.140.A1133
Andrés J, González-Navarrete P, Safont VS (2014) Unraveling reaction mechanisms by means of Quantum Chemical Topology Analysis. Int J Quantum Chem 114:1239–1252
Andres J, Berski S, R. Domingo L, et al (2011) Describing the molecular mechanism of organic reactions by using topological analysis of electronic localization function. Curr Org Chem 15:3566–3575. https://doi.org/10.2174/138527211797636156
Polo V, Andres J, Berski S et al (2008) Understanding reaction mechanisms in organic chemistry from catastrophe theory applied to the electron localization function topology. J Phys Chem A 112:7128–7136. https://doi.org/10.1021/jp801429m
Silvi B, Savin A (1994) Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371:683–686. https://doi.org/10.1038/371683a0
Silvi B (2002) The synaptic order: a key concept to understand multicenter bonding. J Mol Struct 614:3–10. https://doi.org/10.1016/S0022-2860(02)00231-4
Domingo LR, Saéz JA, Zaragozá RJ, Arnó M (2008) Understanding the participation of quadricyclane as nucleophile in polar [2σ + 2σ + 2π] cycloadditions toward electrophilic π molecules. J Org Chem 73:8791–8799. https://doi.org/10.1021/jo801575g
Acknowledgements
The authors wish to acknowledge Dr Louise S. Price, University College London, UK, for reading the manuscript and providing valuable suggestions.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Calculations and optimization of structures were performed by Kosar Norouzi. Analysis, data collection, and writing the first draft of the manuscript were performed by Mina Haghdadi and Mahshid Hamzehloueian and all authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Ethics declarations
Ethics approval
The paper is not currently being considered for publication elsewhere. The paper reflects the authors’ own research and analysis in a truthful and complete manner. No data, text, or theories by others are presented as if they were the author’s own.
Consent to participate
All the authors consent to participate in the research project, and the following has been explained to us: the research may not be of direct benefit to us. My participation is voluntary.
Consent for publication
All the authors approved the version to be published. All the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical responsibilities of authors
We warranty that this manuscript is original, which has been written by the stated authors and has not been published elsewhere; the manuscript has not been submitted to more than one journal for simultaneous consideration. We wish to confirm that it has not been published previously (partly or in full). This study is not split up into several parts. We confirm that no data have been fabricated or manipulated. No data, text, or theories by others are presented as if they were the authors’ own. This manuscript contains no libelous or other unlawful statements and does not contain any materials that violate any personal or proprietary rights of any other person or entity.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haghdadi, M., Norouzi, K. & Hamzehloueian, M. Evaluation of the mechanism, regio-, and diastereoselectivity of aza-Diels–Alder reactions of 2H-azirine under a Lewis acid catalyst. Struct Chem 33, 445–456 (2022). https://doi.org/10.1007/s11224-021-01860-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01860-5