Abstract
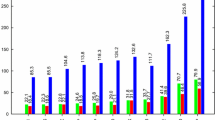
A systematic investigation has been carried out to assess the performance of various exchange–correlation energy density functionals coupled with various basis sets to optimize structural parameters and calculate vibrational frequencies corresponding to 12C5H816O and 12C5H818O isotopomers of 2,3-dihydropyran (DHP) in order to estimate the isotopic shift. For its accurate determination, anharmonic molecular vibrational frequencies have been calculated by employing both, the vibrational self-consistent field (VSCF) method, and the second-order perturbative correction on top of VSCF (VSCF-PT2). We have tested pure DFT GGA functionals (BP86 and PBE), hybrid DFT GGA functionals (B3LYP and X3LYP), pure DFT meta-GGA functionals (TPSS and revTPSS), hybrid DFT meta-GGA functionals (TPSSh and M06), and double hybrid DFT functionals (B2K-PLYP and B2T-PLYP). For the purpose of comparison, the geometry of DHP has been optimized using the MP2 and CCSD methods, and frequencies have been calculated employing MP2 method. From the analysis of all the computed vibrational frequencies, the highest isotopic shift has been found to be ~ 15 cm−1 corresponding to one of the vibrational mode that comprises of =C1−O2 stretch, C3−O2 stretch, and H−C6=C1 bend. Therefore, the present work definitely puts forward the laser-based enrichment process for oxygen-18 isotope separation involving DHP as a working molecule.


Similar content being viewed by others
Availability of data and material
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Lewis GN, Cornish RE (1933) Separation of the isotopic forms of water by fractional distillation. J Am Chem Soc 55:2616–2617. https://doi.org/10.1021/ja01333a518
Huffman JR, Urey HC (1937) Separation of oxygen isotopes by a fractionating column. Ind Eng Chem 29:531–535. https://doi.org/10.1021/ie50329a011
Thode HG, Smith SR, Walkling FO (1944) The separation of the oxygen isotopes by the distillation of water. Can J Res Sec B 22:127–136. https://doi.org/10.1139/cjr44b-016
Dostrovsky I, Hughes ED, Llewellyn DR (1948) Fractional distillation and its application in the concentration of the heavy isotopes of oxygen and hydrogen. Nature 161:858–859. https://doi.org/10.1038/161858a0
Dostrovsky I, Llewellyn DR, Vromen BH (1952) The separation of isotopes by fractional distillation. Part I. Fractionating columns for the enrichment of the heavy isotopes of oxygen in water. J Chem Soc 3509−3517. https://doi.org/10.1039/JR9520003509
Kistemaker J, Bigeleisen J, Nier AOC (eds) (1957) Proceedings of the international symposium on isotope separation. New York, Interscience.
Taylor TI, Bernstein RB (1947) Enrichment of C13 and O18 by a counter-current gaseous exchange process using thermal diffusion. J Am Chem Soc 69:2076–2076. https://doi.org/10.1021/ja01200a523
Ageev EP, Panchenkov GM (1964) The separation of oxygen isotopes by thermal diffusion. Sov At Energy 14:518–520. https://doi.org/10.1007/BF01121902
Kim JW, Choi H−R, Chang D−S, Choi Y−Y (2008) Method for stable oxygen isotope separation and its apparatus using membrane distillation. US 2008/0185283 A1
Kim J, Chang D−S, Choi Y−Y (2009) Separation of oxygen isotopic water by using a pressure-driven air gap membrane distillation. Ind Eng Chem Res 48:5431–5438. https://doi.org/10.1021/ie900277r
Moradi R, Sabet JK, Niassar MS, Amini Y (2016) Air gap membrane distillation for enrichment of H218O isotopomers in natural water using poly (vinylidene fluoride) nanofibrous membrane. Chem Eng Process 100:26–36. https://doi.org/10.1016/j.cep.2015.11.015
Karbasi E, Sabet JK, Rovshandeh JM, Moosavian MA, Ahadi H, Amini Y (2017) Experimental and numerical study of air-gap membrane distillation (AGMD): Novel AGMD module for oxygen-18 stable isotope enrichment. Chem Eng J 322:667–678. https://doi.org/10.1016/j.cej.2017.03.031
Selen NGG, Haydar D, Erhan A, Mahmut E, Filiz K (2018) Enrichment of oxygen-18 isotope by fractional distillation. Turk J Nucl Sci 30:13–23
Pop F, Piciorea I, Iliescu M, Culcer M, Croitoru C, Stefanescu I (2006) Experimental plant for 18o separation by cryogenic oxygen distillation. Electrical Engineering Series 30:399–403
Sander RK, Loree TR, Rockwood SD, Freund SM (1977) ArF laser enrichment of oxygen isotopes. Appl Phys Lett 30:150. https://doi.org/10.1063/1.89313
Marling J (1977) Isotope separation of oxygen-17, oxygen-18, carbon-13, and deuterium by ion laser induced formaldehyde photopredissociation. J Chem Phys 66:4200–4225. https://doi.org/10.1063/1.434496
Zittel PF, Darnton LA, Little DD (1983) Separation of O, C, and S isotopes by two-step, laser photodissociation of OCS. J Chem Phys 79:5991–6005. https://doi.org/10.1063/1.445782
Vizhin VV, Molin YN, Petrov AK, Sorokin AR (1978) Investigations of multiphoton selective dissociation of (ch3)2o in the field of a pulsed co2 laser. Appl Phys 17:385–391. https://doi.org/10.1007/BF00886210
Majima T, Sugita K, Arai S (1989) The 18O separation by IRMPD of ethers. Chem Phys Lett 163:29–33. https://doi.org/10.1016/0009-2614(89)80006-5
Laptev VB, Ryabov EA, Tumanova LM (1995) Results and prospects of laser separation of oxygen isotopes by ir multiphoton dissociation of molecules. Quantum Electron 22:607–614. https://doi.org/10.1070/QE1995v025n06ABEH000425
Kutschke KO, Willis C, Hackett PA (1983) IR Multiphoton decomposition of dimethyl ether. J Photochem 21:207–212. https://doi.org/10.1016/0047-2670(83)80024-0
Laptev VB, Ryabov EA, Tumanova LM (1989) Laser separation of oxygen isotopes by IR multiphoton dissociation of (CH3)2O. Appl Phys B 49:77–83. https://doi.org/10.1007/BF00332130
Mathi P, Kumar A, Ghosh A, Nayak AK, Parthasarathy V, Nataraju V, Jadhav KA, Rajendra Babu K, Sarkar SK (2013) Report No. BARC/2013/E/012.
Sugita K, Majima T, Arai S (1999) 18O-Selective infrared multiple photon decomposition of natural and 18O-enriched diisopropyl ethers. J Phys Chem A 103:4144–4149. https://doi.org/10.1021/jp990222n
Majima T, Igarashi T, Arai S (1984) Infrared multiple-photon decomposition of perfluorodimethyl ether. Nippon Kagaku Kaishi 10:1490–1497
Laptev VB, Tunamova LM, Kuz’menko VA, Ryabov EA (1990) 18O-selective IR MPD of perfluorodimethyl ether. Appl Phys B 51:454–457. https://doi.org/10.1007/BF00329111
Yokoyama A, Katsumata K, Ohba H, Akagi H, Saeki M, Yokoyama K (2008) isotopically selective infrared multiphoton dissociation of 2,3-dihydropyran. J Phys Chem A 112:6571–6577. https://doi.org/10.1021/jp802101h
Hashimoto M, Ohba H, Yokoyama A (2011) Oxygen isotope separation utilizing two-frequency infrared multiphoton dissociation of 2,3-dihydropyran. Appl Phys B 104:969–974. https://doi.org/10.1007/s00340-011-4630-0
Hackett PA, Willis C, Gauthier M (1979) Multiphoton decomposition of hexafluoroacetone: effects of pressure, fluence, wavelength, and temperature on the decomposition yield and C-13 and O-18 isotopic selectivity. J Chem Phys 71:2682–2692. https://doi.org/10.1063/1.438626
Abzianidze TG, Baranov VY, Bakhtadze AB, Belykh D, Vetsko VM, Gurashvili VA, Egiazarov AS, Izyumov SV, Kuz’menko VA, Oziashvili ED et al (1986) Isotopically selective dissociation of cocl2 molecules in the radiation field of a pulsed CO laser. Sov J Quantum Electron 16:137−138. https://iopscience.iop.org/article/10.1070/QE1986v016n01ABEH005250/meta
Takashi K, Hitoshi K (2009) Method and apparatus for enrichment of heavy oxygen isotopes. US2009266702 (A1).
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S et al (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363. https://doi.org/10.1002/jcc.540141112
Wilson AK, Wang NX (2004) The behavior of density functionals with respect to basis set. I. The correlation consistent basis sets. J Chem Phys 121:7632–7646. https://doi.org/10.1063/1.1792071
Wilson AK, Wang NX (2005) Behaviour of density functionals with respect to basis set: II. Polarization consistent basis sets. Mol Phys 103:345–358. https://doi.org/10.1080/00268970512331317264
Mardirossian N, Head-Gordon M (2017) Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 Density Functional. Mol Phys 115:2315–2372. https://doi.org/10.1080/00268976.2017.1333644
Jurečka P, Sponer J, Cerny J, Hobza P (2006) Benchmark database of accurate (MP2 and CCSD(T) complete basis set limit) interaction energies of small model complexes, dna base pairs, and amino acid pairs. Phys Chem Chem Phys 6:1985–1993. https://doi.org/10.1039/B600027D
Bloino J, Biczysko M, Barone V (2012) General perturbative approach for spectroscopy, thermodynamics, and kinetics: methodological background and benchmark studies. J Chem Theory Comput 8:1015–1036. https://doi.org/10.1021/ct200814m
Peterson KA (2009) Gaussian basis sets for quantum mechanical (qm) calculations. Wiley
Peterson KA, Yousaf KE (2010) Molecular core-valence correlation effects involving the post-d elements Ga–Rn: benchmarks and new pseudopotential-based correlation consistent basis sets. J Chem Phys 133:174116. https://doi.org/10.1063/1.3503659
Sylvetsky N, Kesharwani MK, Martin JML (2017) The aug-cc-pVnZ-F12 basis set family: correlation consistent basis sets for explicitly correlated benchmark calculations on anions and noncovalent complexes. J Chem Phys 147:134106. https://doi.org/10.1063/1.4998332
Sylvetsky N, Martin JML (2018) Probing the basis set limit for thermochemical contributions of inner-shell correlation: balance of core-core and core-valence contributions. Mol Phys 117:1078–1087. https://doi.org/10.1080/00268976.2018.1478140
Frisch MJ, Head-Gordon M, Pople JA (1990) A direct mp2 gradient method. Chem Phys Lett 166:275–280. https://doi.org/10.1016/0009-2614(90)80029-D
Hampel C, Peterson K, Werner H-J (1992) A comparison of the efficiency and accuracy of the quadratic configuration interaction (QCISD), coupled cluster (CCSD), and Brueckner coupled cluster (BCCD) Methods. Chem Phys Lett 190:1–12. https://doi.org/10.1016/0009-2614(92)86093-W
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 33:8822–8824, https://doi.org/10.1103/PhysRevB.33.8822; Err. (1986) Phys Rev B 34:7406. https://doi.org/10.1103/PhysRevB.34.7406
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865−3868, https://doi.org/10.1103/PhysRevLett.77.3865 Err. (1997) Phys Rev Lett 78:1396. https://doi.org/10.1103/PhysRevLett.78.1396
Becke AD (1993) A New Mixing of Hartree-Fock and Local Density-Functional Theories. J Chem Phys 98:1372–1377. https://doi.org/10.1063/1.464304
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211. https://doi.org/10.1139/p80-159
Stephens PJ, Devlin FJ, Chablowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. https://doi.org/10.1021/j100096a001
Hertwig RH, Koch W (1997) On the parameterization of the local correlation functional. What is Becke-3-LYP? Chem Phys Lett 268:345–351. https://doi.org/10.1016/S0009-2614(97)00207-8
Xu X, Zhang Q, Muller RP, Goddard WA (2005) An extended hybrid density functional (X3LYP) with improved descriptions of nonbond interactions and thermodynamic properties of molecular systems. J Chem Phys 122:014105. https://doi.org/10.1063/1.1812257
Perdew JP, Tao J, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta–generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146401. https://doi.org/10.1103/PhysRevLett.91.146401
Perdew JP, Tao J, Staroverov VN, Scuseria GE (2004) Meta-generalized gradient approximation: explanation of a realistic nonempirical density functional. J Chem Phys 120:6898–6911. https://doi.org/10.1063/1.1665298
Perdew JP, Ruzsinsky A, Csonka GI, Constantin LA, Sun J (2009) Workhorse semilocal density functional for condensed matter physics and quantum chemistry. Phys Rev Lett 103:026403, https://doi.org/10.1103/PhysRevLett.103.026403; Err. (2011) Phys Rev Lett 106:179902. https://doi.org/10.1103/PhysRevLett.106.179902
Staroverov VN, Scuseria GE, Tao J, Perdew JP (2003) Comparative assessment of a new nonempirical density functional: molecules and hydrogen-bonded complexes. J Chem Phys 119:12129−12137, https://doi.org/10.1063/1.1626543; Err. (2004) J Chem Phys 121:11507−11507. https://doi.org/10.1063/1.1795692
Zhao Y, Truhlar DG (2008) The M06 Suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functional. Theoret Chem Acc 120:215−241, https://doi.org/10.1007/s00214-007-0310-x; Err. (2008) Theoret Chem Acc 119:525. https://doi.org/10.1007/s00214-007-0401-8
Tarnopolsky A, Karton A, Sertchook R, Vuzman D, Martin JML (2008) Double-hybrid functionals for thermochemical kinetics. J Phys Chem A 112:3–8. https://doi.org/10.1021/jp710179r
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function-augmented basis sets for anion calculations. III. The 3–21+G Basis Set for First-Row Elements, Li–F. J Comput Chem 4:294–301. https://doi.org/10.1002/jcc.540040303
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/B508541A
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023. https://doi.org/10.1063/1.456153
Baldridge KK, Gordon MS, Steckler R, Truhlar DG (1989) Ab initio reaction paths and direct dynamics calculations. J Phys Chem 93:5107–5119. https://doi.org/10.1021/j100350a018
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
Bowman JM (1978) Self-consistent field energies and wavefunctions for coupled oscillators. J Chem Phys 68:608–610. https://doi.org/10.1063/1.435782
Gerber RB, Ratner MA (1979) A semiclassical self-consistent field (SC SCF) approximation for eigenvalues of coupled-vibration systems. Chem Phys Lett 68:195–198. https://doi.org/10.1016/0009-2614(79)80099-8
Jung JO, Gerber RB (1996) Vibrational wave functions and spectroscopy of (H2O)n, n=2,3,4,5: vibrational self-consistent field with correlation corrections. J Chem Phys 105:10332–10348. https://doi.org/10.1063/1.472960
Roy TK, Carrington T Jr, Gerber RB (2014) Approximate first-principles anharmonic calculations of polyatomic spectra using MP2 and B3LYP potentials: comparisons with experiment. J Phys Chem A 118:6730–6739. https://doi.org/10.1021/jp5060155
Roy TK, Sharma R, Gerber RB (2016) First-principles anharmonic quantum calculations for peptide spectroscopy: VSCF calculations and comparison with experiments. Phys Chem Chem Phys 18:1607–1614. https://doi.org/10.1039/c5cp05979h
Roy TK, Kopysov V, Pereverzev A, Šebek J, Gerber RB, Boyarkin OV (2018) Intrinsic structure of pentapeptide leu-enkephalin: geometry optimization and validation by comparison of VSCF-PT2 calculations with cold ion spectroscopy. Phys Chem Chem Phys 20:24894–24901. https://doi.org/10.1039/c8cp03989e
Mitra H, Roy TK (2020) Comprehensive benchmark results for the accuracy of basis sets for anharmonic molecular vibrations. J Phys Chem A 124:9203–9221. https://doi.org/10.1021/acs.jpca.0c06634
López JC, Alonso JL (1985) The microwave spectrum of 3,4-dihydro-1,2-pyran. Z Naturforsch. 40a:913−919. https://doi.org/10.1515/zna-1985-0909
Ocola EJ, Brito T, McCann K, Laane J (2010) Conformational energetics and low-frequency vibrations of cyclohexene and its oxygen analogs. J Mol Struct 978:74–78. https://doi.org/10.1016/j.molstruc.2009.11.026
Cordero B, Gómez V, Platero-Prats AE, Revés M, Echeverría J, Cremades E, Barragán F, Alvarez S (2008) Covalent radii revisited. Dalton Trans 2832–2838. https://doi.org/10.1039/B801115J
Pyykkö P, Atsumi M (2009) Molecular single-bond covalent radii for elements 1–118. Chem Eur J 15:186–197. https://doi.org/10.1002/chem.200800987
Pyykkö P, Atsumi M (2009) Molecular double-bond covalent radii for elements Li–E112. Chem Eur J 15:12770–12779. https://doi.org/10.1002/chem.200901472
Pyykkö P (2015) Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: a summary. J Phys Chem A 119:2326–2337. https://doi.org/10.1021/jp5065819
Lord RC, Rounds TC, Ueda T (1972) Far infrared spectra of ring compounds. X. hindered pseudorotation in six‐membered rings: estimation of the barrier height for half‐chair inversion in dioxene and 2,3‐dihydropyran. J Chem Phys 57:2572−2580, https://doi.org/10.1063/1.1678625; Err. (1975) J Chem Phys 62:754. https://doi.org/10.1063/1.431017
Tecklenburgt MMJ, Laane J (1989) Vibrational potential energy surfaces of 3,4-dihydro-2H-pyran, 3,6-dihydro-2H-pyran, 2,3-dihydro-1,4-dioxin, and 4H–1,3-dioxin. J Am Chem Soc 111:6920–6926. https://doi.org/10.1021/ja00200a005
Durig JR, Carter RO, Carreira LA (1974) Raman spectra of gases. XII. Twisting mode and barriers to planarity and interconversion in 2,3-dihydropyran, 1,4-dioxene, and cyclohexene. J Chem Phys 60:3098–3103. https://doi.org/10.1063/1.1681495
Bushweller CH, O’Neil JW (1969) Conformational isomerism in dihydropyran. Conjugative stabilization of the half-chair conformer. Tetrahedron Lett 53:4713–4716. https://doi.org/10.1016/S0040-4039(01)88791-8
Garcia D, Grunwald E (1980) Pulsed IR laser study of half-chair to boat interconversion of 2,3-dihydropyran. J Am Chem Soc 102:6407–6411. https://doi.org/10.1021/ja00541a003
Shishkina SV, Shishkin OV, Leszczynski J (2002) Three-stage character of ring inversion in cyclohexene. Chem Phys Lett 354:428–434. https://doi.org/10.1016/S0009-2614(02)00156-2
Shishkin OV, Shishkina SV (2011) Unusual properties of usual molecules. Conformational analysis of cyclohexene, its derivatives and heterocyclic analogues, In: J. Leszczynski and M. Shukla (eds.), Practical aspects of computational chemistry i. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0919-5_19
Manzhos S, Carrington T, Laverdure L, Mosey N (2015) Computing the anharmonic vibrational spectrum of UF6 in 15 dimensions with an optimized basis set and rectangular collocation. J Phys Chem A 119:9557–9567. https://doi.org/10.1021/acs.jpca.5b07627
Rivera-Gaines VE, Leibowitz SJ, Laane J (1991) far-infrared spectra, two-dimensional vibrational potential energy surface, and conformation of cyclohexene and its isotopomers. J Am Chem Soc 113:9735–9742. https://doi.org/10.1021/ja00026a004
Tecklenburg MMJ, Laane J (1989) Vibrational potential energy surfaces of 3,4-dihydro-2H-pyran, 3,6-dihydro-2H-pyran, 2,3-dihydro-1,4-dioxin, and 4H–1,3-dioxin. J Am Chem Soc 111:6920–6926. https://doi.org/10.1021/ja00200a005
Dorofeeva OV (1992) Ideal gas thermodynamic properties of oxygen heterocyclic compounds: Part 2. Six-membered, seven-membered and eight-membered rings. Thermochim Acta 200:121−150. https://doi.org/10.1016/0040-6031(92)85111-8; and NIST/EPA Gas-Phase Infrared Database.
Boatz A, Gordon MS (1989) Decomposition of normal-coordinate vibrational frequencies. J Phys Chem 93:1819–1826. https://doi.org/10.1021/j100342a026
Chesnokov EN, Gorelik SR, Gritsan NP (2003) Calculations of the Isotopic Shifts of Fundamental Frequencies for Dihaloid Silanes. Vib Spectrosc 32:241–248. https://doi.org/10.1016/S0924-2031(03)00065-1
Acknowledgements
The authors gratefully acknowledge the generous support provided by their host institution, Bhabha Atomic Research Centre, Mumbai. The authors would like to thank the Computer Division, Bhabha Atomic Research Centre for providing computational facilities. It is a pleasure to thank Dr. Tapan K. Ghanty, Mr. M. Mascarenhas, and Dr. Archana Sharma for their kind interest and encouragements.
Author information
Authors and Affiliations
Contributions
Conceptualization, JPN; methodology, AG; formal analysis and investigation, AG; writing (original draft preparation), AG; writing (review and editing), JPN.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghosh, A., Jonnalgadda, P.N. Ab initio and DFT benchmark study for the calculations of isotopic shifts of fundamental frequencies for 2,3-dihydropyran. Struct Chem 33, 743–755 (2022). https://doi.org/10.1007/s11224-021-01829-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01829-4