Abstract
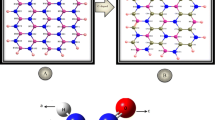
A theoretical study has been conducted onto the pristine, Nb-, and Au-doped boron nitride (BN) nanosheets using DFT calculations with the B3LYP-D3 method in order to evaluate their stabilities and electronic properties. The interaction of the guanine molecule with these clusters was also examined in order to determine their adsorption properties. The calculations show that the HOMO-LUMO energy gap (Eg) of the BN nanosheet was strongly decreased upon its doping with Nb and Au atoms, implying a strong enhancement in its surface reactivity. The interaction of the guanine with the BN nanosheet was found to be weak, which leads a slight variation in its energy gap; therefore, a low sensitivity of this nanosheet toward the guanine molecule was observed. The guanine adsorption over the NbBN cluster is very strong, and the calculated adsorptions energies are in the range of −36.7 to −60.2 kcal mol−1, suggesting a great chemical adsorption. For the AuBN cluster, the guanine molecule has been chemisorbed onto its surface with adsorption energies which vary from −24.2 to −38.4 kcal mol−1, which are lower than those obtained for the NbBN cluster. Upon adsorption process, the energy gap of the NbBN cluster was greatly increased, which leads to a decrease in its electrical conductivity; thereby, it cannot be a suitable sensor for the detection of the guanine molecule. On the contrary, the energy gap of the AuBN cluster was reduced by the effect of the guanine adsorption on its surface, indicating an increase in its electrical conductivity; thus, the AuBN cluster possesses a great electronic sensitivity to the guanine molecule. Based on the transition state theory, the recovery time of the guanine desorption from the AuBN cluster was estimated of 27.6 s, reflecting that the Au-doped BN nanosheet could be employed as an appropriate nanosensor for the guanine molecule detection with a short recovery time.








Similar content being viewed by others
Data availability
Data can be obtained through the corresponding author from email.
References
Schmid G (1992) Large clusters and colloids. Metals in the embryonic state. Chem Rev 92:1709–1727
Mori K, Miyawaki K, Yamashita H (2016) Ru and Ru-Ni nanoparticles on TiO support as extremely active catalysts for hydrogen production from ammonia borane. ACS Catal 6:3128–3135
Mokrane T, Boudjahem A, Bettahar M (2016) Benzene hydrogenation over alumina-supported nickel nanoparticles prepared by polyol method. RSC Adv 6:59858–59864
Boudjahem A, Mokrane T, Redjel A, Bettahar M (2010) Silica supported nanopalladium prepared by hydrazine reduction. C R Chimie 13:1433–1439
Liu A, Xu Y, Qiu X, Huang C, Liu M (2019) Chemoselective hydrogenation of nitrobenzenes activated with tuned au/h-BN. J Catal 370:55–60
Boudjahem A, Bettahar M (2017) Effect of oxidative pre-treatment on hydrogen spillover for a Ni/SiO2 catalyst. J Mol Catal A 24:190–197
Redjel A, Boudjahem A, Bettahar M (2018) Effect of palladium precursor and preparation method on the catalytic performance of Pd/SiO2 catalysts for benzene hydrogenation. Particul Sci Technol 36:710–715
Chen N, Zhu Z, Su T, Liao W, Deng C, Ren W, Zhao Y, Lu H (2020) Catalytic hydrogenolysis of hydroxymethylfurfural to highly selective 2,5-dimethylfuran over FeCoNi/h-BN catalyst. Chem Engin J 381:122755
Boudjahem A, Monteverdi S, Mercy M, Bettahar M (2004) Nanonickel particles supported on silica. Morphology effects on their surface and hydrogenating properties. Catal Lett 97:177–183
Boudjahem A, Redjel A, Mokrane T (2012) Preparation, characterization and performance of Pd/SiO2 catalyst for benzene catalytic hydrogenation. J Ind Chem Eng 18:303–308
Kon K, Onodera W, Toyao T, Shimizu K (2016) Supported rhenium nanoparticle catalysts for acceptoless dehydrogenation of alcohols: structure-activity relationship and mechanistic studies. Catal Sci Technol 6:5864–5870
Shen J, Yang L, Hu K, Luo W, Cheng G (2015) Rh nanoparticles on graphene as efficient catalyst for hydrolytic dehydrogenation of amine boranes for chemical hydrogen storage. Int J Hydrog Energy 40:1062–1070
Wang J, Zhang X, Wang Z, Wang L, Zhang Y (2012) Rhodium-nickel nanoparticles grown on graphene as highly efficient catalyst for complete decomposition of hydrous hydrazine at room temperature for chemical hydrogen storage. Energy Environ Sci 5:6885–6888
Dong L, Sanganna Gari RR, Li Z, Craig M, Hou S (2010) Graphene-supported platinium and platinium-ruthenium nanoparticles with high electrocatalytic activity for methanol and ethanol oxidation. Carbon. 48:781–787
Qiu X, Wu X, Wu Y, Liu Q, Huang C (2016) The release hydrogen from ammounia borane over copper/hexagonal boron nitride composites. RSC Adv 6:106211–106217
Shen H, Duan C, Guo J, Zhao N, Xu J (2015) Facile in situ synthesis of silver nanoparticles on boron nitride nanosheets with enhanced catalytic performance. J Mater Chem 3:16663–16669
Goyal A, Aggarwal D, Kapoor S, Goel N, Singhal S, Shukla J (2020) A comprehensive experimental and theoretical study on BN nanosheets for the adsorption of pharmaceutical drugs. New J Chem 44:3985–3997
Yang H, Gu S, Li J, Jin L, Xie X, Luo L, Xiao J, Li J, Li C, Chen Y (2021) Synthesis of boron carbonitride nanosheets using for delivering paclitaxel and their antitumor activity. Colloid Surf B 198:111479
Zhang Y, Guo R, Wang D, Sun X, Xu Z (2019) Pd nanoparticle-decorated hydroxy boron nitride nanosheets as a novel drug carrier for chemo-photothermal therapy. Colloid Surf B 176:300–308
Du M, Liu Q, Huang C, Qiu X (2017) One-step synthesis of magnetically recyclable co@BN core-sheel nanocatalysts for catalytic reduction of nitroarenes. RSC Adv 7:35459–35459
Huang C, Ye W, Liu Q, Qiu X (2014) Dispersed Cu2O octahedrons on h-BN nanosheets for p-nitrophenol reduction, ACS Appl. Mater. Interfaces. 6:14469–14476
Fu Q, Meng Y, Fang Z, Hu Q, Xu L, Gao W, Huang X, Xue Q, Sun Y, Lu F (2017) Boron nitride nanosheet-anchored Pd-Fe core-shell nanoparticles as highly efficient catalysts for Suzuki-Miyaura coupling reactions, ACS Appl. Mater Interfaces 9:2469–2476
Huang C, Chen C, Ye X, Ye W, Hu J, Xu C, Qiu X (2013) Stable colloidal boron nitride nanosheet dispersion and its potential application in catalysis. J Mater Chem A 1:12192
Yang XJ, Li LL, Sang WL, Zhao JL, Wang XX, Yu C, Zhang XH, Tang CC (2017) Boron nitride supported Ni nanoparticles as catalysts for hydrogen generation from hydrolysis of ammounia borane. J Alloy Compound 693:642–649
Behmagham F, Vessally E, Massoumi B, Hosseinian A, Edjlali L (2016) A computational study on the SO2 adsorption by the pristine, Al, and Si doped BN nanosheets. Superllatices Microstructures 100:350–357
Zhao P, Su Y, Zhang Y, Li SJ, Chen G (2011) CO catalytic oxidation on iron-embedded hexagonal boron nitride sheet. Chem Phys Lett 515:159–161
Esrafili MD (2018) NO reduction by CO molecule over Si-doped boron nitride nanosheet: a dispersion-corrected DFT study. Chem Phys Lett 695:131–137
Lee JH, Choi YK, Kim HJ, Scheicher R, Cho JH (2013) Physisorption of DNA nucleobaseson h-BN and graphene: vdW-corrected DFT calculations. J Phys Chem A 117:13435–13441
Lin Q, Zou X, Zhou G, Liu R, Wu J, Li J, Duan W (2011) Adsorption of DNA/RNA nucleobases on hexagonal boron nitride sheet: an ab initio study. Phys Chem Chem Phys 13:12225–12230
Singla P, Riyaz M, Singhal S, Goel N (2016) Theoretical study of adsorption of amino acids on graphene and BN sheet in gas and aqueous phase including empirical DFT dispersion correction. Phys Chem Chem Phys 18:5597–5604
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Jr JA, Montgomery JE, Peralta F, Ogliaro M, Bearpark JJ, Heyd E, Brothers KN, Kudin VN, Staroverov T, Keith R, Kobayashi J, Normand K, Raghavachari A, Rendell JC, Burant SS, Iyengar J, Tomasi M, Cossi N, Rega JM, Millam M, Klene JE, Knox JB, Cross V, Bakken C, Adamo J, Jaramillo R, Gomperts RE, Stratmann O, Yazyev AJ, Austin R, Cammi C, Pomelli JW, Ochterski RL, Martin K, Morokuma VG, Zakrzewski GA, Voth P, Salvador JJ, Dannenberg S, Dapprich AD, Daniels O, Farkas JB, Foresman JV, Ortiz J, Cioslowski DJ (2013) Fox, Gaussian 09, Revision D.01. Gaussian, Inc., Wallingford
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Becke AD (1993) Density-functional thermochemistry. III The role of exact exchange J Chem Phys 98:5648–5652
Lee C, Yang W, Parr R (1988) LYP correlation functional. Phys Rev B 37:785
Charkin OP, Klimenko NM, Charkin DO (2019) DFT modeling of successive hydrogenated subnano-size aluminum clusters. Chem Phys 522:112–122
Rad SA, Esfahanian M, Maleki S, Gharati G (2016) Application of carbon nanostructures toward SO2 and SO3 adsorption: a comparison between pristine graphene and N-doped graphene by DFT calculations. J Sulf Chem 37:176–188
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to au including the outermost core orbitals. J Chem Phys 82:299–310
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654
Soltani A, Boudjahem A (2014) Stabilities, electronic and magneticproperties of small Rhn (n=2–12) clusters: a DFT approach. Comput Theor Chem 1047:6–14
Padash R, Nasrabadi M, Rad AS, Nasab AS (2018) Jesionowski T., H. Ehrlich, a comparative computational investigation of phosgene adsorption on (XY)12 (X = Al, B and Y = N, P) nanoclusters: DFT investigations. J Clust Sci 30:203–218
Karaman A, Boudjahem A, Boulbazine M, Gueid A (2020) Stability and electronic properties of IrnV (n = 2–10) nanoclusters and their reactivity toward N2H4 molecule. Struct Chem 31:203–214
Boulbazine M, Boudjahem A, Chaguetmi S, Karaman A (2020) Stability and electronic properties of Rh-doped ruthenium clusters and their interaction with NH3 molecule. Mol Phys 118:e1643511
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comput Chem 20:129–154
Ersanm F, Gokoglu G, Akturk E (2014) Bimetallic two-dimensional PtAg coverage on h-BN substrate: first-principles calculations. Appl Surf Sci 303:306–311
Xu D, Liu YJ, Zhao JX, Cai QH, Wang XZ (2014) Theoeretical study of the deposition of Pt clusters on defective hexagonal boron nitride (h-BN) sheets: morphologies, electronic structures, and interactions with O. J Phys Chem C 118:8868–8876
Solozhenko VL, Lazarenko AG, Petitet JP, Kanaev AV (2001) Band gap energy of graphite-like hexagonal boron nitride. J Phys Chem Solids 62:1331–1334
Bouderbala W, Boudjahem A, Soltani A (2014) Geometries, stabilities, electronic and magnetic properties of small PdnIr (n = 1–8) clusters from first-principles calculations. Mol Phys 112:1789–1798
Soltani A, Boudjahem A, Bettahar M (2016) Electronic and magnetic properties of small RhnCa (n = 1-9) clusters: a DFT study. Int J Quantum Chem 5:346–356
Pansini FN, Campos M, Neto AC, Sergio CS (2020) Theoretical study of the electronic structure and electrical properties of Al-doped niobium clusters. Chem Phys 535:110778
Cheng S, Sun X, Zhao L, Chen J (2019) The interaction of guanine nucleobase with B40 borospherene. Eur Phys J D 73:88
Code availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2843 kb)
Rights and permissions
About this article
Cite this article
Derdare, M., Boudjahem, AG. Adsorption of the guanine molecule over the pristine, Nb-, and Au-doped boron nitride nanosheets: a DFT study. Struct Chem 32, 2159–2173 (2021). https://doi.org/10.1007/s11224-021-01785-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01785-z