Abstract
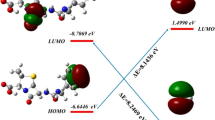
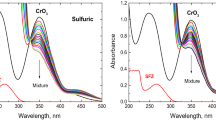
The antibiotic para-aminosalicylic acid (PAS) is decomposed into its decarboxylated product, meta-aminophenol, with a series of toxicity symptoms including hemolytic anemia. The present trial investigates the decarboxylation mechanism of PAS, along with an in-detail assessment of the potential energy profiles, geometries, kinetic, and thermodynamic preference of the process. Further, the effects of solvent and pH on the process have been fully investigated. B3LYP-D3(BJ)/G4MP2 calculations have been executed on the para-aminosalicylic acid decomposition with a view to clarify the aqueous thermal decarboxylation and pH influences on the mechanism. The decomposition process can only be described accurately by the inclusion of solvent not only as a reaction medium but also as an active contributor to the chemical process. We further found an optimal preference with respect to energy barriers for the process with decreasing pH. Also, experiments showed the reduction in PAS absorbance with fall in pH. These together suggested the decrease of rate of decarboxylation with pH increase. Finally, we concluded that increasing pH reduces the reaction rate and in the pH around 10, the reaction rate reaches zero and it completely halts. We assume that this experiment can pave the way for advanced experimental and theoretical researches in the drug degradation mechanisms field and the effect of pH and solvent on the process.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
Tao LJ, Chen WT, Jing L, Ji Q, Ren JL (2017) A network pharmacology approach to establish the pharmacological mechanism of JiaWeiXianJiTang on inflammatory bowel disease. Biomed Rep 6(3):272–278
Dasgupta Q, Madras G, Chatterjee K (2016) Controlled release kinetics of p-aminosalicylic acid from biodegradable crosslinked polyesters for enhanced anti-mycobacterial activity. Acta Biomater 30:168–176
Vetuschi C, Ragno G, Mazzeo P (1988) Determination of p-aminosalicylic acid and m-aminophenol by derivative UV-spectrophotometry. J Pharm Biomed Anal 6(4):383–391
Kornblum SS, Sciarrone BJ (1964) Decarboxylation of p-aminosalicylic acid in the solid state. J Pharm Sci 53(8):935–941
Pande GS (1967) Thermal decarboxylation. J Sci Ind Res India 26(9):393
Anderson DMW, Garbutt S (1963) 597. Studies on uronic acid materials. Part VII. The kinetics and mechanism of the decarboxylation of uronic acids. J Chem Soc 3204–3210
Jivani SG, Stella VJ (1985) Mechanism of decarboxylation of p-aminosalicylic acid. J Pharm Sci 74(12):1274–1282
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Chem Phys 98(45):11623–11627
Pritchard BP, Altarawy D, Didier B, Gibson TD, Windus TL (2019) New basis set exchange: an open, up-to-date resource for the molecular sciences community. J Chem Inf Model 59(11):4814–4820
Zahedi E, Shaabani S, Shiroudi A (2017) Following the molecular mechanism of decarbonylation of unsaturated cyclic ketones using bonding evolution theory coupled with NCI analysis. J Phys Chem A 121(44):8504–8517
Li X, Frisch MJ (2006) Energy-represented direct inversion in the iterative subspace within a hybrid geometry optimization method. J Chem Theory Comput 2(3):835–839
Jacobsen H, Bérces A, Swerhone DP, Ziegler T (1997) Analytic second derivatives of molecular energies: a density functional implementation. Comput Phys Commun 100(3):263–276
Bérces A, Dickson RM, Fan L, Jacobsen H, Swerhone D, Ziegler T (1997) An implementation of the coupled perturbed Kohn-Sham equations: perturbation due to nuclear displacements. Comput Phys Commun 100(3):247–262
Fukui K (1981) The path of chemical reactions-the IRC approach. Acc Chem Res 14(12):363–368
Fukui K (1970) Formulation of the reaction coordinate. J Phys Chem 74(23):4161–4163
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory using reduced order perturbation theory. J Chem Phys 127(12):124105
Narayanan B, Redfern PC, Assary RS, Curtiss LA (2019) Accurate quantum chemical energies for 133000 organic molecules. Chem Sci 10(31):7449–7455
Hadidi S, Shiri F, Norouzibazaz M (2020) Theoretical mechanistic insight into the gabapentin lactamization by an intramolecular attack: Degradation model and stabilization factors. J Pharm Biomed 178:112900
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102(11):1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24(6):669–681
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev Comput Mol Sci 2(1):73–78
Marvin 20.20 (2020) ChemAxon (http://www.chemaxon.com)
Poater J, Fradera X, Duran M, Solà M (2003) The delocalization index as an electronic aromaticity criterion: application to a series of planar polycyclic aromatic hydrocarbons. Chem Eur J 9(2):400–406
Poater J, Duran M, Sola M, Silvi B (2005) Theoretical evaluation of electron delocalization in aromatic molecules by means of atoms in molecules (AIM) and electron localization function (ELF) topological approaches. Chem Rev 105(10):3911–3947
Kruszewski J, Krygowski TM (1972) Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett 13(36):3839–3842
Krygowski T (1993) Crystallographic studies of inter-and intramolecular interactions reflected in aromatic character of. pi.-electron systems. J Chem Inf Comput Sci 33(1):70–78
Dapprich S, Frenking G (1995) Investigation of donor-acceptor interactions: a charge decomposition analysis using fragment molecular orbitals. J Phys Chem 99(23):9352–9362
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Rodriquez C, Williams I (1997) Ring strain energy and enthalpy of formation of oxiranone: an ab initio theoretical determination. J Chem Soc, Perkin Trans 2 5: 953-958
Author information
Authors and Affiliations
Contributions
• N. Hemati: Niloofar Hemati conceived of the presented idea and supervised the project.
• F. Shiri: Farshad Shiri planned and carried out the simulations.
• S. Hadidi: Saba Hadidi planned and carried out the simulations.
• E. Mohammadi: Elham Mohammadi took the lead in writing the manuscript.
• R. Parvizi: Rasool Parvizi took the lead in writing the manuscript.
• M. H. Farzaeie: Mohammad Hosein Farzaeie supervised the project.
• All authors provided critical feedback and helped shape the research, analysis, and manuscript.
• All authors drafted the work or revised it critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interests
Consent to publish
All authors approved the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical approval
1) This material (partially or in full) is the authors’ own original work, which has not been previously published elsewhere.
2) The paper is not currently being considered for publication elsewhere.
3) The paper reflects the authors’ own research and analysis in a truthful and complete manner.
4) No data, text, or theories by others are presented as if they were the author’s own.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. PAS is decomposed into its decarboxylation product, meta-aminophenol.
2. The solvent is not only as a reaction medium but further it acts as an active contributor in the chemical process
3. The process shows a significant rate reduction by increasing pH.
Supplementary information
ESM 1
(DOCX 19393 kb).
Rights and permissions
About this article
Cite this article
Hemati, N., Shiri, F., Hadidi, S. et al. A theoretical investigation on decarboxylation mechanism of antibiotic para-aminosalicylic acid to highly toxic form meta-aminophenol. Struct Chem 32, 1053–1060 (2021). https://doi.org/10.1007/s11224-020-01676-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01676-9