Abstract
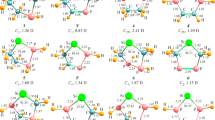
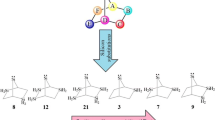
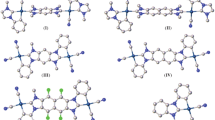
Following our quest for stable group 14 divalents, novel N-heterocyclic plumbylenes (NHPbs), composed of 2,4,6-cycloheptatriene-2,7-diazaplumbylene (1), benzannulated with one (2), two (3, 4), and three benzene rings (5), are compared and contrasted at the density functional theory level. Results indicate that in going from 1 to 5, the absolute values of singlet-triplet energy gap (ΔEs-t) and band gap (ΔEH-L) increase, while nucleophilicity (N), electrophilicity (ω), and chemical potential (μ) decrease. The most benzannulated structure, ([a,c,e]tribenzo)cyclohepta-2,7-diazaplumbylene (5), turns out as the most stable plumbylene for showing the most negative ΔEs-t of − 194.94 kcal/mol. Furthermore, 5 shows the highest ΔEH-L (− 2.75 eV), with the lowest N (1.31 eV), ω (8.20 eV), μ (− 7.15 eV), and charge of (+ 0.736) on Pb atom. Isodesmic reactions of 1-5 with common transition metal halides, MX2, give forty new metal complexes of 1M-X-5M-X, where M = Pt and Pd, while X = F, Cl, Br, and I. Their complexation energies (ΔECom) indicate that 1-5 are rather stronger ligands with Pt than Pd. Bader’s atoms in molecules (AIM) and NBO analyses show the partial covalent and partial electrostatic nature of Pb-M bonds.

We have scrutinized novel N-heterocyclic plumbylenes (NHPbs: 1-5), by DFT. Structure 5 turns out as the most stable plumbylene for showing the highest ΔEs-t. It shows the highest ΔEH-L with the lowest N, ω, μ, and charge on Pb atom. The results show that NHPbs form stronger complexes with Pt atom than Pd.






















Similar content being viewed by others
References
Petz W (1986). Chem Rev 86:1019–1047
Tokitoh N, Okazaki R (2000). Coord Chem Rev 210:251–277
Mizuhata Y, Sasamori T, Tokitoh N (2009). Chem Rev 109:3479–3511
Baumgartner J, Marschner C (2014). Rev Inorg Chem 34:119–152
Zabula AV, Hahn FE (2008). Eur J Inorg Chem 2008:5165–5179
Krupski S, Pöttgen R, Schellenberg I, Hahn FE (2014). Dalt Trans 43:173–181
Dasgupta R, Das S, Hiwase S, Pati SK, Khan S (2019). Organometallics 38:1429–1435
Schneider J, Sindlinger CP, Freitag SM, Schubert H, Wesemann L (2017). Angew Chemie 129:339–343
Wu Y, Shan C, Sun Y, Chen P, Ying J, Zhu J, Liu LL, Zhao Y (2016). Chem Commun 52:13799–13802
Hadlington TJ, Hermann M, Frenking G, Jones C (2014). J Am Chem Soc 136:3028–3031
Heitmann D, Pape T, Hepp A, Mück-Lichtenfeld C, Grimme S, Hahn FE (2011). J Am Chem Soc 133:11118–11120
Arp H, Baumgartner J, Marschner C, Zark P, Müller T (2012). J Am Chem Soc 134:10864–10875
Braunschweig H, Celik MA, Dewhurst RD, Heid M, Hupp F, Sen SS (2015). Chem Sci 6:425–435
Tapu D, Dixon DA, Roe C (2009). Chem Rev 109:3385–3407
Arduengo III AJ, Kline M, Calabrese JC, Davidson F (1991). J Am Chem Soc 113:9704–9705
Denk M, Lennon R, Hayashi R, West R, Belyakov AV, Verne HP, Haaland A, Wagner M, Metzler N (1994). J Am Chem Soc 116:2691–2692
Herrmann WA, Denk M, Behm J, Scherer W, Klingan F, Bock H, Solouki B, Wagner M (1992). Angew Chemie 104:1489–1492
Gans-Eichler T, Gudat D, Nieger M (2002). Angew Chemie Int Ed 41:1888–1891
Charmant JPH, Haddow MF, Hahn FE, Heitmann D, Fröhlich R, Mansell SM, Russell CA, Wass DF (2008). Dalt Trans:6055–6059
Hahn FE, Heitmann D, Pape T (2008). Eur J Inorg Chem 2008:1039–1041
Connor EF, Nyce GW, Myers M, Möck A, Hedrick JL (2002). J Am Chem Soc 124:914–915
Jeong W, Hedrick JL, Waymouth RM (2007). J Am Chem Soc 129:8414–8415
Chang YA, Waymouth RM (2017). J Polym Sci Part A Polym Chem 55:2892–2902
Melaimi M, Soleilhavoup M, Bertrand G (2010). Angew Chemie Int Ed 49:8810–8849
Hahn FE, Jahnke MC (2008). Angew Chemie Int Ed 47:3122–3172
Hill NJ, West R (2004). J Organomet Chem 689:4165–4183
Zabula AV, Hahn FE, Pape T, Hepp A (2007). Organometallics 26:1972–1980
Kim SB, Sinsermsuksakul P, Pike RD, Gordon RG (2014). Chem Mater 26:3065–3073
Parameswaran P, Frenking G (2009). Chem Eur J 15:8807–8816
Hupp F, Ma M, Kroll F, Jimenez-Halla JOC, Dewhurst RD, Radacki K, Stasch A, Jones C, Braunschweig H (2014). Chem Eur J 20:16888–16898
Lin JCY, Huang RTW, Lee CS, Bhattacharyya A, Hwang WS, Lin IJB (2009). Chem Rev 109:3561–3598
Böhm VPW, Weskamp T, Gstöttmayr CWK, Herrmann WA (2000). Angew Chemie Int Ed 39:1602–1604
Sanford MS, Love JA, Grubbs RH (2001). J Am Chem Soc 123:6543–6554
Muehlhofer M, Strassner T, Herrmann WA (2002). Angew Chemie Int Ed 41:1745–1747
Zhong F, Yang X, Shen L, Zhao Y, Ma H, Wu B, Yang X-J (2016). Inorg Chem 55:9112–9120
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993). J Comput Chem 14:1347–1363
Gordon MS, Schmidt MW (2005) Theory and applications of computational chemistry. Elsevier, pp 1167–1189
Domingo LR, Pérez P (2011). Org Biomol Chem 9:7168–7175
Chattaraj PK, Giri S, Duley S, Phys J (2011). Chem A 116:790–791
Smit B, Frenkel D (1989). Mol Phys 68:951–958
Glendening ED, Landis CR, Weinhold F (2012) Wiley Interdiscip. Rev Comput Mol Sci 2:1–42
Ayoubi-Chianeh M, Kassaee MZ, Ashenagar S, Cummings PT (2019). J Phys Org Chem:e3956
Ayoubi-Chianeh M, Kassaee MZ (2019). Res Chem Intermed 45:4677–4691
Takagi N, Frenking G (2011). Theor Chem Accounts 129:615–623
Acknowledgments
Special thanks are due to Seyed Abdolreza Miran, Maniya Sadat Miran, and Seyed Manny Miran for their continued encouragement and moral support.
Funding
We gratefully acknowledge Tarbiat Modares University for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 8836 kb)
Rights and permissions
About this article
Cite this article
Mohebi, N., Kassaee, M.Z. New N-heterocyclic plumbylenes (NHPbs) and their complexes with palladium and platinum by DFT. Struct Chem 32, 731–757 (2021). https://doi.org/10.1007/s11224-020-01603-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01603-y