Abstract
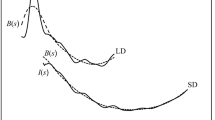
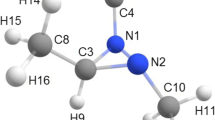
The molecular structure of thiosalicylamide (2-hydroxythiobenzamide) was investigated in the gas phase at 401 K by means of gas electron diffraction (GED) combined with quantum chemical (QC) calculations. Special attention was paid to the internal rotation of the thioamide group. Structural refinement was performed taking into account rovibrational corrections to the thermal-average internuclear distances calculated with harmonic and anharmonic (cubic) MP2/cc-pVTZ force constants in terms of static and dynamic models. It was shown that both models fitted the GED data equally well. The results of the GED refinement revealed that in the equilibrium structure, the thioamide group is twisted by about 30° with respect to the phenol ring plane. This is the result of an interatomic repulsion of hydrogen atom in the amide group from the closest hydrogen atom of the benzene ring, which overcomes the energy gain from the π−π conjugation of the thioamide group and the aromatic system of thiosalicylamide. Natural bond orbital (NBO) analysis and comparison of the thiosalicylamide molecular structure with those of related compounds revealed hydrogen-bonded fragment between the hydroxyl and thiocarbonyl groups. The structure of thiosalicylamide in the gas phase was found to be markedly different from that in the solid phase due to the effect of intermolecular hydrogen bonding in the crystal.










Similar content being viewed by others
References
Pospisilova S, Michnova H, Kauerova T, Pauk K, Kollar P, Vinsova J, Imramovsky A, Cizek A, Jampilek J (2018). Bioorg Med Chem Lett 28(12):2184–2188. https://doi.org/10.1016/j.bmcl.2018.05.011
Ueda J, Khan ST, Takagi M, Shin-ya K (2010). J Antibiot 63(5):267–269. https://doi.org/10.1038/ja.2010.26
Hardie DG (2013). Diabetes 62(7):2164–2172. https://doi.org/10.2337/db13-0368
Mehanna AS, Kim JY (2005). Bioorg Med Chem 13(13):4323–4331. https://doi.org/10.1016/j.bmc.2005.04.012
Zhang Y, Mantravadi PK, Jobbagy S, Bao W, Koh JT (2016). ACS Chem Biol 11(10):2797–2802. https://doi.org/10.1021/acschembio.6b00659
Palomar J, De Paz JLG, Catalán J (1999). Chem Phys 246(1–3):167–208. https://doi.org/10.1016/s0301-0104(99)00159-7
Pertlik F (1990). Monatsh Chem 121:129–139. https://doi.org/10.1007/BF00809525
Velcheva EA, Stamboliyska BA (2008). J Mol Struct 875(1–3):264–271. https://doi.org/10.1016/j.molstruc.2007.04.038
Anandan K, Kolandaivel P, Kumaresan R (2005). Int J Quantum Chem 104(3):286–298. https://doi.org/10.1002/qua.20559
Manin AN, Voronin AP, Perlovich GL (2013). Thermochim Acta 551:57–61. https://doi.org/10.1016/j.tca.2012.10.013
Aarset K, Page EM, Rice DA (2013). J Phys Chem A 117(14):3034–3040. https://doi.org/10.1021/jp311003d
Banerjee K, Raychaudhury S (1982). Bull Chem Soc Jpn 55(11):3621–3624. https://doi.org/10.1246/bcsj.55.3621
Sambathkumar K (2015). Spectrochim Acta A 147:51–66. https://doi.org/10.1016/j.saa.2015.03.052
Jezierska A, Panek JJ, Mazzarello R (2009). Theor Chem Accounts 124(5–6):319–330. https://doi.org/10.1007/s00214-009-0612-2
Briel D (2005). Heterocycles 65(6):1295–1309
Kochikov IV, Kovtun DM, Tarasov YI (2008). Num Meth Program 9:12–18 http://num-meth.srcc.msu.ru/zhurnal/tom_2008/pdf/v9r202.pdf. Accessed 13.09.2010
Frish MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann JR, Burant JC, DapprichS, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komazomi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PM, Johnson B, Chen W, Wong MW, Andres JL, Gonzales C, Head-Gordon M, Replogle ES, Pople JA (2003) Gaussian 03 (Revision D01). Gaussian Inc., Pittsburgh
Becke AD (1988). Phys Rev A 38(6):3098–3000. https://doi.org/10.1103/PhysRevA.38.3098
Lee C, Yang W, Parr RG (1988). Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Møller C, Plesset MS (1934). Phys Rev 46:618–622. https://doi.org/10.1103/PhysRev.46.618
Petersson GA, Bennett A, Tensfeldt TG, Al-Laham MA, Shirley WA, Mantzaris J (1988). J Chem Phys 89:2193–2218. https://doi.org/10.1063/1.455064
Dunning TH (1989). J Chem Phys 90:1007–1023. https://doi.org/10.1063/1.456153
Weinhold F, Landis CR (2001). Chem Educ Res Pract 2:91–104. https://doi.org/10.1039/B1RP90011K
G. A. Zhurko, D. A. Zhurko, ChemCraft 1.6 build vol. 332 https://www.chemcraftprog.com/index.html. Accessed 20.06.2010
Vishnevskiy YV, UNEX, 2007, version 1.5. http://unexprog.org. Accessed 2.09.2013
Ischenko AA, Girichev GV, Tarasov YI (2013) Electron diffraction: structure and dynamics of free molecules and condensed state of substance. Fizmatlit, Moscow
Sipachev VA (2000). Struct Chem 11:167–172. https://doi.org/10.1023/A:1009217826943
Vishnevskiy YV, Zhabanov YA (2015). J Phys Conf Ser 633:012076. https://doi.org/10.1088/1742-6596/633/1/012076
Tikhonov DS, Vishnevskiy YV, Rykov AN, Grikina OE, Khaikin LS (2017). J Mol Struct 1132:20–27. https://doi.org/10.1016/j.molstruc.2016.05.090
Kolesnikova IN, Putkov AE, Rykov AN, Shishkov IF (2018). J Mol Struct 1161:76–82. https://doi.org/10.1016/j.molstruc.2018.01.084
Portalone G, Schultz G, Domenicano A, Hargittai I (1992). Chem Phys Lett 197:482–488. https://doi.org/10.1016/0009-2614(92)85804-J
Pauling L (1960) The nature of the chemical bond3rd edn. Cornell University Press, Ithaca
Wiberg KB (1968). Tetrahedron 24:1083–1196. https://doi.org/10.1016/0040-4020(68)88057-3
Acknowledgments
The authors express their gratitude to Dr. Ilya I. Marochkin from Lomonosov Moscow State University, Arseniy A. Otlyotov, Dr. Yury A. Zabanov, and Prof. Nina I. Giricheva from Ivanovo State University of Chemistry and Technology for valuable consultations which were very useful for preparing this manuscript.
Funding
This project was made with financial support of the Russian Foundation for Basic Research (Grant Number 18-33-00546 mol_a).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 712 kb)
Rights and permissions
About this article
Cite this article
Kolesnikova, I.N., Rykov, A.N., Shuvalov, M.V. et al. Internal rotation and intramolecular hydrogen bonding in thiosalicylamide: gas phase electron diffraction study supported by quantum chemical calculations. Struct Chem 30, 1993–2001 (2019). https://doi.org/10.1007/s11224-019-01369-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01369-y