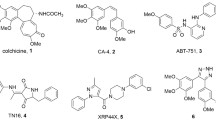
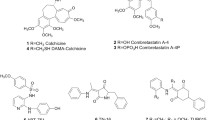
Abstract
Four novel conjugates of adamantane connected at a bridgehead position via four-bond linker to structurally different ligands interacting with colchicine binding site of tubulin were synthesized. All compounds were found to inhibit cancer cell proliferation and, notably, to promote the formation of different atypical tubulin assemblies — clusters, curly, or “entangled” microtubules. The distinction in the observed effects for conceptually equivalent structural templates can be partially explained on the basis of computer molecular modeling.






Similar content being viewed by others
References
Hadfield JA, Ducki S, Hirst N, McGown AT (2003) Tubulin and microtubules as targets for anticancer drugs. Prog Cell Cycle Res 5:309–325
Risinger AL, Giles FJ, Mooberry SL (2009) Microtubule dynamics as a target in oncology. Cancer Treat Rev 35:255–261
Karecla P, Hirst E, Bayley P (1989) Polymorphism of tubulin assembly in vitro. J Cell Sci 94:479–488
Yvon AM, Wadsworth P, Jordan MA (1999) Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell 10:947–959
Gupta S, Bhattacharyya B (2003) Antimicrotubular drugs binding to vinca domain of tubulin. Mol Cell Biochem 253:41–47
Perez EA (2009) Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther 8:2086–2095
Saltarelli D, Pantaloni D (1982) Polymerization of the tubulin-colchicine complex and guanosine 5′-triphosphate hydrolysis. Biochemistry 21:2996–3006
Zefirova ON, Lemcke H, Lantow M, Nurieva EV, Wobith B, Onishchenko GE, Hoenen A, Griffiths G, Zefirov NS, Kuznetsov SA (2013) Unusual tubulin-clustering ability of definitely C7-modified colchicine analogues. ChemBioChem 14:1444–1449
Zefirov NA, Hoppe M, Kuznetsova IV, Chernyshov NА, Grishin YK, Maloshitskaya OA, Kuznetsov SA, Zefirova ON (2018) Homologues series of novel adamantane – colchicine conjugates: synthesis and cytotoxic effect on human cancer cells. Mendeleev Commun 28:225–344
Thomopoulou P, Sachs J, Teusch N, Mariappan A, Gopalakrishnan J, Schmalz HG (2015) New colchicine-derived triazoles and their influence on cytotoxicity and microtubule morphology. ACS Med Chem Lett 7:188–191
Vilanova C, Díaz-Oltra S, Murga J, Falomir E, Carda M, Redondo-Horcajo JM, Díaz JF, Barasoain I, Marco A (2014) Design and synthesis of pironetin analogue/colchicine hybrids and study of their cytotoxic activity and mechanisms of interaction with tubulin. J Med Chem 57:10391–10403
Zefirova ON, Nurieva EV, Wobith B, Gogol VV, Zefirov NA, Ogonkov AV, Shishov DV, Zefirov NS, Kuznetsov SA (2017) Novel antimitotic agents related to tubuloclustin: synthesis and biological evaluation. Mol Divers 21:547–564
Nurieva EV, Zefirov NA, Mamaeva AV, Wobith B, Kuznetsov SA, Zefirova ON (2018) Synthesis of steroid analogs of tubuloclustin, their cytotoxicity and effect on microtubules of A549 carcinoma cells. Russ Chem Bull 67:688–693
Nguyen TL, McGrath C, Hermone AR, Burnett JC, Zaharevitz DW, Day BW, Wirf P, Hamel E, Gussio R (2005) A common pharmacophore for a diverse set of colchicine site inhibitors using a structure-based approach. J Med Chem 48:6107–6116
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Al-Haddad A, Shonn MA, Redlich B, Blocker A, Burkhardt JK, Yu H, Hammer 3rd JA, Weiss DG, Steffen W, Griffiths G, Kuznetsov SA (2001) Myosin Va bound to phagosomes binds to F-actin and delays microtubule-dependent motility. Mol Biol Cell 12:2742–2755
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock 4 and AutoDockTools 4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Bagnato JD, Eilers AL, Horton RA, Grissom CB (2004) Synthesis and characterization of a cobalamin-colchicine conjugate as a novel tumor-targeted cytotoxin. J Org Chem 69:8987–8996
Funding
This work was supported by Russian Fund of Fundamental Research (project no. №18-03-00524) and the German organization DAAD (German Academic Exchange Service) under the auspices of a collaborative agreement between Moscow and Rostock Universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zefirov, N.A., Evteeva, Y.A., Wobith, B. et al. Adamantyl-substituted ligands of colchicine binding site in tubulin: different effects on microtubule network in cancer cells. Struct Chem 30, 465–471 (2019). https://doi.org/10.1007/s11224-018-1219-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1219-9