Abstract
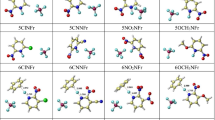
The prominent roles of organofluorine compounds in various fields have aroused considerable interest in the development of new methods for carbon-fluorine bond formation. Electrophilic fluorination receives much attention. Density functional theory (DFT) was used to theoretically explore the fluorination activity of 12 N-Fluoropyridinium salts on the substrate of benzene in acetonitrile solution. Geometry optimizations and frequency calculations of the reactants, transition states, and products were performed at B3LYP/6-311G(d,p) level for the 12 fluorination reaction channels. Based on the optimized structure, all the stationary points have been corrected by the single point energy at a high-level of M06-2x/6-311++G (d,p). Four substituents were considered in this paper, they are nitro-, cyano-, chloro-, methoxy-, respectively. Three substituted sites (ortho-, meso-, para-) were also concluded. Based on the obtained potential energy surfaces information and analysis of substituent effect, the fluorination reaction channel of oNO2NFpyr is most efficient due to the lowest reaction energy barrier; therefore, oNO2NFpyr is a promising optimum fluorinating reagent among the studied 12 N-Fluoropyridinium salts.



Similar content being viewed by others
References
Chambers RD (2004) Fluorine in organic chemistry, Blackwell Publishing Ltd, pp 1–22
Uneyama K (2006) Organofluorine chemistry. Blackwell, Oxford, p 3
Banks RE, Smart BE, Tatlow JC (1994) Organofluorine chemistry: principles and commercial application. Plenum Press, New York
Dong CJ, Huang FL, Deng H, Schaffrath C, Spencer JB, O’Hagan D, Naismith JH (2004) Crystal structure and mechanism of a bacterial fluorinating enzyme. Nature 427:561–565
O’Hagan D, Deng H (2015) Enzymatic fluorination and biotechnological developments of the fluorinase. Chem Rev 115:634–649
Umemoto T, Fukami S, Tomizawa G, Harasawa K, Kawada K, Tomita K (1990) Power- and structure-variable fluorinating agents. The N-fluoropyridinium salt system. J Am Chem Soc 112:8563–8575
Differding E, Lang RW (1988) New fluorinating reagents-I. The first enantioselective fluorination reaction. Tetrahedron Lett 29:6087–6090
Liu W, Huang XY, Cheng MJ, Robert JN, Goddard III WA, Groves JT (2012) Oxidative aliphatic C-H fluorination with fluoride ion catalyzed by a manganese porphyrin. Science 337:1322
Steven B, Cody RP, David CM, Nathan H, Maxwell GH, Ellen U, Thomas L (2012) A polycomponent metal-catalyzed aliphatic, allylic, and benzylic fluorination. Angewandtechemie 124:10732–10735
Cody RP, Steven B, Ryan W, Dillon JA, Lev RR, Maxime AS, Thomas L (2014) Direct, catalytic monofluorination of sp3 C-H bonds: a radical-based mechanism with ionic selectivity. J Am Chem Soc 136:9780–9791
Yin F, Wang ZT, Li ZD, Li CZ (2015) Silver-catalyzed decarboxylative fluorination of aliphatic carboxylic acids in aqueous solution. J Am Chem Soc 80:5834–5841
Satoshi M, Ida SRS, O’Duill M, Jamie W, Anna KK, Sarita JF, Matthew T, Graham S, Peter RM, Mickael H, Sajinder KL, Jan P, Olof S, Véronique G (2013) Catalytic decarboxylative fluorination for the synthesis of tri- and difluoromethyl arenes. Org Lett 15:2648–2651
Lin JH, Xiao JC (2014) Recent advances in asymmetric fluorination and fluoroalkylation reactions via organocatalysis. Tetrahedron let 55:6147–6155
Wolstenhulme JR, Gouverneur V (2014) Asymmetric fluorocyclizations of alkenes. Acc Chem Res 47:3560–3570
Lee SY, Neufeind S, Fu GC (2014) Nantioselective nucleophile-catalyzed synthesis of tertiary alkyl fluorides via the α-fluorination of ketenes: synthetic and mechanistic studies. J Am Chem Soc 136:8899–8902
Egami H, Asada J, Sato K, Hashizume D, Kawato Y, Hamashima Y (2015) Asymmetric fluorolactonization with a bifunctional hydroxyl carboxylate catalyst. J Am Chem Soc 137:10132–10135
Xue XS, Wang Y, Li M, Cheng JP (2016) Comprehensive energetic scale for quantitatively estimating the fluorinating potential of N-F reagents in electrophilic fluorinations. J Org Chem 81:4280–4289
Geng CH, Du LK, Liu F, Zhu RX, Liu CB (2015) Theoretical study on the mechanism of selective fluorination of aromatic compounds with Selectfluor. RSC Adv 5:33385–33391
Gennady IB, Pavel AZ, Vyacheslav GS (2006) Electrophilic fluorination of aromatic compounds with NF type reagents: kinetic isotope effects and mechanism. Tetrahedron Lett 47:2639–2642
Borodkin GI, Zaikin PA, Shakirov MM, Shubin VG (2007) Mechanism of electrophilic fluorination of aromatic compounds with NF-reagents. Russ J Org Chem 43:1451–1459
Becke AD (1993) Becke’s three parameter hybrid method using the LYP correlation functional. J Chem Phys 98:5648–5652
Lee C, Wang W, Parr RG (1988). Phys Rev 37:785–789
Gonzalez G, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phy Chem B 113:6378–6396
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.-A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox (2009) Gaussian 09 (Revision A. 02): Gaussian Inc., Wallingford, CT
Hammond GS (1955) A correlation of reaction rates. J Am Chem Soc 77:334–338
Acknowledgements
We thank the grid computing server provided by the Chinese Academy of Sciences.
Funding
This work is supported by the National Basic Research Program of China (2012CB723308), the National Natural Science Foundation of China (51337002 and 50977019), the Doctoral Foundation by the Ministry of Education of China (20112303110005), and the Science Foundation for Distinguished Young Scholar of Heilongjiang Province (JC201206).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
The geometry coordinates of reactants, transition states and products of twelve fluorination reactions optimized at B3LYP/6-311G(d,p)level (DOC 98 kb)
Rights and permissions
About this article
Cite this article
Du, X., Zhang, H., Yao, Y. et al. Theoretical study on the fluorination of benzene with N-Fluoropyridinium salts in acetonitrile solution. Struct Chem 29, 1601–1607 (2018). https://doi.org/10.1007/s11224-018-1135-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1135-z