Abstract
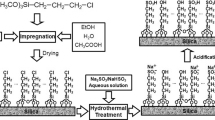
Silica-supported Pd catalysts were synthesized in the presence of the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. Two samples with extremely low Pd loadings, 0.35Pd and 0.08Pd, with Pd contents 0.35 and 0.08%, respectively, were subjected to further investigations. Structural characterization was performed by ICP-AES and Raman measurements. Raman spectra indicated the presence of the ionic liquid in the Pd-silica samples. The samples were tested as catalysts in the Heck coupling reactions of methyl acrylate and styrene, with substituted bromoarenes and chloroarenes. Both samples proved to be highly efficient catalysts and displayed excellent activities and selectivities for the reactions of activated haloarenes including chloroarenes, which could be efficiently transformed without applying harsh reaction conditions. As expected, the presence of an electron withdrawing group (EWG) on the aromatic ring of the haloarene was found to increase both the conversion and the selectivity to an appreciable extent. For the transformations of bromoarenes, the sample with the lowest Pd loading proved to be a more efficient catalyst. Upon recycling of the catalysts, a considerable activity loss was detected, which was attributed to an extensive leaching of Pd into the solution, as confirmed by hot filtration measurements.








Similar content being viewed by others
References
Johansson CCC, Kitching MO, Colacot TJ, Snieckus V (2012) Angew Chem Int Ed 51:5062–5085
Molnár Á (2011) Chem Rev 111:2251–2320
Elazab HA, Siamaki AR, Moussa S, Gupton BF, El-Shall MS (2015) Appl Catal A Gen 491:58–69
Wang P, Zhang G, Jiao H, Liu I, Deng X, Chen Y, Zheng X (2015) Appl Catal A Gen 489:188–192
Zhao F, Murakami K, Shirai M, Arai M (2000) J Catal 194:479–483
Reetz MT, de Vries JG (2004) Chem Commun 1559–1563
Rocaboy C, Gladysz JA (2002) Org Lett 4:1993–1996
Martinez AV, Mayoral JA, Garcia JI (2014) Appl Catal A Gen 472:21–28
Amatore C, Jutand A (2000) Acc Chem Res 33:314–321
Polshettiwar P, Len C, Fihri A (2009) Coord Chem Rev 253:2599–2626
Köhler K, Wussow K, Wirth AS (2013) In: Molnár Á (ed) Palladium-catalyzed coupling reactions: practical aspects and future developments. Wiley-VCH, Weinheim
Stouten SC, Noel T, Wang Q, Hessel V (2015) Chem Eng J 279:143–148
Garrett C, Prasad K (2004) Adv Synth Catal 346:889–900
Cole-Hamilton DJ (2003) Science 299:1702–1706
Beletskaya I, Cheprakov A (2000) Chem Rev 100:3009–3066
Papp A, Miklós K, Forgó P, Molnár Á (2005) J Mol Catal A Chem 229:107–116
Reetz MT, Westermann E, Lohmer R, Lohmer G (1998) Tetrahedron Lett 39:8449–8452
Yao Q, Kinney EP, Yang Z (2003) J Org Chem 68:7528–7531
Polshettiwar V, Molnár Á (2007) Tetrahedron 63:6949–6976
Mastalir Á, Quiroga M, Szabó T, Zsigmond Á, Dékány I (2014) React Kinet Mech Catal 113:61–68
Beller M, Fischer H, Kühlein K, Reisinger CP, Herrmann WA (1996) J Organomet Chem 520:257–259
Djakovitch L, Koehler K (2001) J Am Chem Soc 123:5990–5999
Yuranov I, Moeckli P, Suvorova E, Buffat P, Kiwi-Minsker L, Renken A (2003) J Mol Catal A Chem 192:239–251
Calo V, Nacci A, Monopoli A, Fornaro A, Sabbatini L, Cioffi N, Ditaranto N (2004) Organometallics 23:5154–5158
Corma A (1997) Chem Rev 97:2373–2419
Taguchi A, Schüth F (2005) Micropor Mesopor Mat 77:1–45
Ying JY, Mehnert CP, Wong MS (1999) Angew Chem Int Ed 38:56–77
Wight AP, Davis ME (2002) Chem Rev 102:3589–3613
Anwander R (2001) Chem Mater 13:4419–4438
On DT, Desplantier-Giscard D, Danumah C, Kaliaguine S (2003) Appl Catal A Gen 253:545–602
Panpranot J, Pattamakomsan K, Goodwin JG, Praserthdam P (2004) Catal Commun 5:583–590
Selvam P, Mohapatra SK, Sonavane SU, Jayaram RV (2004) Appl Catal B Environ 49:251–255
Mathews CJ, Smith PJ, Welton T, White AJP, Williams DJ (2001) Organometallics 20:3848–3850
Zhao D, Fei Z, Geldbach TJ, Scopelliti R, Dyson PJ (2004) J Am Chem Soc 126:15876–15882
Hagiwara H, Sugawara Y, Isobe K, Hoshi T, Suzuki T (2004) Org Lett 6:2325–2328
Okubo K, Shirai M, Yokoyama C (2002) Tetrahedron Lett 43:7115–7118
Shi F, Zhang Q, Li D, Deng Y (2005) Chem Eur J 11:5279–5288
Heidenreich RG, Köhler K, Krauter JGE, Pietsch J (2002) Synlett 1118–1122
Jeffery T (1996) Tetrahedron 52:10113–10130
Consorti CS, Flores FR, Dupont J (2005) J Am Chem Soc 127:12054–12065
Reetz MT, Westermann E (2000) Angew Chem Int Ed 39:165–168
Zhao FY, Shirai M, Ikushima Y, Arai M (2002) J Mol Catal A Chem 180:211–219
Köhler K, Heidenreich RG, Krauter JGE, Pietsch J (2002) Chem Eur J 8:622–631
Pröckl SS, Kleist W, Gruber MA, Köhler K (2004) Angew Chem Int Ed 43:1881–1882
Widegren JA, Finke RG (2003) J Mol Catal A Chem 198:317–341
Ananikov VP, Beletskaya IP (2012) Organometallics 31:1595–1604
Sigeev AS, Peregudov AS, Cheprakov AV, Beletskaya IP (2015) Adv Synth Catal 357:417–429
Acknowledgements
Financial support from the Hungarian National Science Foundation through OTKA Grant K 109278 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bucsi, I., Mastalir, Á., Molnár, Á. et al. Heck coupling reactions catalysed by Pd particles generated in silica in the presence of an ionic liquid. Struct Chem 28, 501–509 (2017). https://doi.org/10.1007/s11224-016-0892-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0892-9