Abstract
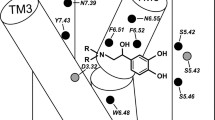
Dexmedetomidine (4-[(S)-1-(2,3-dimethyl-phenyl)-ethyl]-1H-imidazole), Dex, is potent agonist acting on α2-adrenergic receptors (α2-ARs). It can exist at the physiological pH in both forms: neutral and protonated. The results of receptor-independent and receptor-dependent studies applied to both forms of Dex are reported. A conformational analysis with PM3 semiempirical MO and ab initio HF/6-31G* methods was carried out for both forms of Dex. The calculated geometries of low-energy conformers of Dex were compared with X-ray geometry and those of conformers resulted from molecular docking of Dex in the binding pockets of 3D homology models of the α2A-, α2B-, and α2C-adrenoceptor subtypes. A MM/QM (molecular mechanics/quantum mechanics) docking study was performed to refine and optimize receptor–ligand complex and close contacts between the ligand and amino acids lining the binding cavity. Two-dimensional potential energy surface and docking results suggest that the imidazole ring can easily adopt the best orientation for an efficient interaction with the carboxylate group of Asp3.32 from the binding cavity of alpha2 adrenergic receptor subtypes.







Similar content being viewed by others
References
Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP et al (2004) Dexmedetomidine produces its neuroprotective effect via the α2A-adrenoceptor subtype. Eur J Pharmacol 502:87–97
Carollo DS, Nossaman BD, Ramadhyani U (2008) Dexmedetomidine: a review of clinical applications. Curr. Opin. Anaesthesiol. 21:457–461
Gyires K, Zádori ZS, Török T, Mátyus P (2009) α2-Adrenoceptor subtypes-mediated physiological, pharmacological actions. Neurochem Int 55:447–453
Gentili F, Pigini M, Piergentili A, Giannella M (2007) Agonists and antagonists targeting the different alpha2-adrenoceptor subtypes. Curr Top Med Chem 7:163–186
Crassous P-A, Denis C, Paris H, Sénard JM (2007) Interest of alpha2-adrenergic agonists and antagonists in clinical practice: background, facts and perspectives. Curr Top Med Chem 7:187–194
HyperChem, version 7.52 release for Windows, 2006, HyperCube, Inc., Gainsville
Stewart JJP (1989) Optimization of parameters for semiempirical methods I. Method. J Comput Chem 10:209–220
Cherezov V, Rosenbaum DM, Hanson MA, Søren GF, Thian FS, Kobilka TS et al (2007) High resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318:1258–1265
Ostopovici-Halip L, Curpăn R, Mracec M, Bologa CG (2011) Structural determinants of the alpha2 adrenoceptor subtype selectivity. J Mol Graph Model 29:1030–1038
Ostopovici-Halip L, Borota A, Mracec M, Curpan R, Gruia A, Mracec M (2009) 3D homology model of the α2A-adrenergic receptor subtype. Rev Roum Chim 54:157–161
Ostopovici-Halip L, Borota A, Gruia A, Mracec M, Rad-Curpan R, Mracec M (2010) 3D homology model of the α2B-adrenergic receptor subtype. Rev Roum Chim 55:343–348
Halip L, Gruia AT, Borota A, Mracec M, Curpan RF, Mracec M (2012) 3D homology model of the α2C-adrenergic receptor subtype. Rev Roum Chim 57:763–768
LigPrep, version 2.4, 2010, Schrödinger, LLC, New York, NY
Maestro, version 9.1, 2010, Schrödinger, LLC, New York, NY
Protein Preparation Wizard, 2010, Schrödinger LLC, New York, NY
Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 27:221–234
Schrödinger Release 2010-1: SiteMap, version 1.7, Schrödinger, LLC, New York, NY, 2010.
Halgren T (2007) New method for fast and accurate binding-site identification and analysis. Chem Biol Drug Des 69:146–148
Halgren TA (2009) Identifying and characterizing binding sites and assessing druggability. J Chem Inf Model 49:377–389
Wang CD, Buck MA, Fraser CM (1991) Site-directed mutagenesis of alpha 2A-adrenergic receptors: identification of amino acids involved in ligand binding and receptor activation by agonists. Mol Pharmacol 40:168–179
Sherman W, Day T, Jacobson MP, Friesner RA, Farid R (2006) Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem 49:534–553
Glide version 5.6, 2010, Schrödinger, LLC, New York, NY
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA et al (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 49:6177–6196
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759
Prime version 2.2, 2010, Schrödinger, LLC, New York, NY
Jacobson MP, Pincus DL, Rapp CS, Day TJF, Honig B, Shaw DE et al (2004) A hierarchical approach to all-atom protein loop prediction. Proteins. 55:351–367
Jacobson MP, Friesner RA, Xiang Z, Honig B (2002) On the role of the crystal environment in determining protein side-chain conformations. J Mol Biol 320:597–608
QSite version 5.6, 2010, Schrödinger, LLC, New York, NY
Murphy RB, Philipp DM, Friesner RA (2000) Mechanics (QM/MM) Method for Large-Scale Modeling of Chemistry in Protein Environments. J Comput Chem 21:1442–1457
Philipp DM, Friesner RA (1999) Mixed ab initio QM r MM modeling using dipeptide and tetrapeptide. J Comput Chem 20:1468–1494
Horner-Miller B (2006) In: Proceedings of the 2006 ACM/IEEE conference on supercomputing. ACM, Tampa, p 746
Guo Z, Mohanty U, Noehre J, Sawyer TK, Sherman W, Krilov G (2010) Probing the alpha-helical structural stability of stapled p53 peptides: molecular dynamics simulations and analysis. Chem Biol Drug Des 75(4):348–359
Shivakumar D, Williams J, Wu Y, Damm W, Shelley J, Sherman W (2010) Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. J Chem Theory Comput 6(5):1509–1519
Schrödinger Release 2015-1: Desmond molecular dynamics system, version 4.1, D. E. Shaw Research, New York, NY, 2015. Maestro-Desmond Interoperability Tools, version 4.1, Schrödinger, New York, NY, 2015
Kivikoski JH, Rissanen KT, Parhi SSL (1993) Crystal structures and absolute configurations of dexmedetomidine and its tosyl derivative. Tetrahedron Asymmetry 4:45–58
Acknowledgments
This work was supported by CNCSIS-UEFISCSU, project number PN-II-PCE-ID 1268/2007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Curpăn, R.F., Halip, L., Borota, A. et al. Modeling of dexmedetomidine conformers and their interactions with alpha2 adrenergic receptor subtypes. Struct Chem 27, 871–881 (2016). https://doi.org/10.1007/s11224-015-0645-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0645-1