Abstract
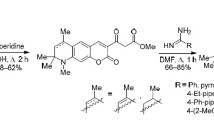
A reaction of 2-(4,4,6-trimethyl-2-oxo-4H-pyrrolo[3,2,1-ij]quinoline-1-ylidene)-hydrazinocarbothioamides with α-halocarbonyl compounds, such as ethyl bromocetate and 2-bromoacetophenone derivatives, afforded a series of novel 4,4,6-trimethyl-2-oxo-4H-pyrrolo[3,2,1-ij]quinoline-1(2H)-ylidene)hydrazinylidene)thiazolidin-4-ones and 4,4,6-trimethyl-1-(2-(4-arylthiazol-2-yl)hydrazinylidene)-4H-pyrrolo[3,2,1-ij]quinolin-2(1H)-ones. The synthesized compounds exist in the form of Z-isomers. Primary screening in vitro of inhibitory activity towards blood clotting factors Xa and XIa was carried out, revealing that the thiazole derivative bearing the 4-chlorophenyl substituent at the thiazole moiety exhibits a sufficiently high anticoagulant activity towards these blood clotting factors.
Similar content being viewed by others
References
G. I. Zhungietu, M. A. Rekhter, Izatin i yego proizvodnyye [Isatin and its Derivatives], Shtiintsa, Kishinev, 1977, 228 p.
J. F. M. da Silva, S. J. Garden, A. C. Pinto, J. Braz. Chem. Soc., 2001, 12, 273; DOI: https://doi.org/10.1590/S0103-50532001000300002.
S. N. Pandeya, S. Smitha, M. Jyoti, S. K. Sridhar, Acta Pharm., 2005, 55, 27.
B. Bhrigu, D. Pathak, N. Siddiqui, M. S. Alam, W. Ahsan, Int. J. Pharm. Sci. Drug Res., 2010, 2, 229.
Ye. V. Leshcheva, S. M. Medvedeva, Kh. S. Shikhaliev, Zhurnal organichnoi ta farmatsevtichnoi khimii [Journal of Organic and Pharmaceutical Chemistry], 2014, 12, 15 (in Russian).
N. P. Novichikhina, A. S. Shestakov, A. Yu. Potapov, E. A. Kosheleva, G. V. Shatalov, V. N. Verezhnikov, D. Yu. Vandyshev, I. V. Ledeneva, Kh. S. Shikhaliev, Russ. Chem. Bull., 2020, 69, 787; DOI: https://doi.org/10.1007/s11172-020-2834-3.
I. Ilin, E. Lipets, A. Sulimov, D. Kutov, Kh. Shikhaliev, A. Potapov, M. Krysin, F. Zubkov, L. Sapronova, F. Ataullakhanov, V. Sulimov, J. Mol. Graph. Mod., 2019, 89, 215; DOI: https://doi.org/10.1016/j.jmgm.2019.03.017.
V. B. Sulimov, I. V. Gribkova, M. P. Kochugaeva, E. V. Katkova, A. V. Sulimov, D. C. Kutov, Kh. S. Shikhaliev, S. M. Medvedeva, M. Yu. Krysin, E. I. Sinauridze, F. I. Ataullakhanov, BioMed Res. Int., 2015, Art. ID 120802; DOI: https://doi.org/10.1155/2015/120802.
S. M. Medvedeva, A. Yu. Potapov, I. V. Gribkova, E. V. Katkova, V. B. Sulimov, Kh. S. Shikhaliev, Pharm. Chem. J., 2018, 51, 975; DOI: https://doi.org/10.1007/s11094-018-1726-4.
N. Novichikhina, I. Ilin, A. Tashchilova, A. Sulimov, D. Kutov, I. Ledenyova, M. Krysin, Kh. Shikhaliev, A. Gantseva, E. Gantseva, N. Podoplelova, V. Sulimov, Molecules, 2020, 25, 1889; DOI: https://doi.org/10.3390/molecules25081889.
N. P. Novichikhina, A. A. Skoptsova, A. S. Shestakov, A. Yu. Potapov, E. A. Kosheleva, O. A. Kozaderov, I. V. Ledenyova, N. A. Podoplelova, M. A. Panteleev, Kh. S. Shikhaliev, Russ. J. Org. Chem., 2020, 56, 1550; DOI: https://doi.org/10.1134/S1070428020090080.
M. Gümüş, M. Yakan, İ. Koca, Future Med. Chem., 2019, 11, 1979; DOI: https://doi.org/10.4155/fmc-2018-0196.
M. H. M. Helal, M. A. Salem, M. S. A. El-Gaby, M. Aljahdali, Eur. J. Med. Chem., 2013, 65, 517; DOI: https://doi.org/10.1016/j.ejmech.2013.04.005.
R. N. Sharma, F. P. Xavier, K. K. Vasu, S. C. Chaturvedi, S. S. Pancholi, J. Enzyme Inhib. Med. Chem., 2009, 24, 890; DOI: https://doi.org/10.1080/14756360802519558.
S. B. Srinivasa, B. Poojary, U. Brahmavara, A. J. Das, S. K. Middha, ChemistrySelect, 2018, 3, 12478; DOI: https://doi.org/10.1002/slct.201801398.
M. A. T. Nguyen, A. K. Mungara, J.-A. Kim, K. D. Lee, S. Park, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 191; DOI: https://doi.org/10.1080/10426507.2014.914933.
P. F. da S. Santos-Junior, I. J. dos S. Nascimento, E. C. D. da Silva, K. L. C. Monteiro, J. D. de Freitas, S. de Lima Lins, M. T. de Aquino, New J. Chem., 2021, 45, 13847; DOI: https://doi.org/10.1039/d1nj02105b.
Y. A. Ammar, A. M. El-Sharief, Y. A. Mohamed, A. B. Mehany, A. Ragab, Al-Azhar Bull. Sci., 2018, 29, 25; DOI: https://doi.org/10.21608/ABSB.2018.33767.
N. Siddiqui, W. Ahsan, Eur. J. Med. Chem., 2010, 45, 1536; DOI: https://doi.org/10.1016/j.ejmech.2009.12.062.
F. Song, Zh. Li, Y. Bian, X. Huo, J. Fang, L. Shao, M. Zhou, Arch. Pharm., 2020, 353, 2000143; DOI: https://doi.org/10.1002/ardp.202000143.
Y.-J. Qin, P.-F. Wang, J. A. Makawana, Z.-C. Wang, Z.-N. Wang, G. Yan, A.-Q. Jiang, H.-L. Zhu, Bioorg. Med. Chem. Lett., 2014, 24, 5279; DOI: https://doi.org/10.1016/j.bmcl.2014.09.054.
M. F. Abo-Ashour, W. M. Eldehna, R. F. George, M. M. Abdel-Aziz, M. M. Elaasser, N. M. A. Gawad, A. Gupta, S. Bhakta, S. M. Abou-Seri, Eur. J. Med. Chem., 2018, 160, 49; DOI: https://doi.org/10.1016/j.ejmech.2018.10.008.
P. Hoffmann, A. Bernat, P. Savi, J. M. Herbert, J. Pharmacol. Exp. Ther., 1998, 286, 670.
A. E. Amr, N. M. Sabrry, M. M. Abdalla, B. F. Abdel-Wahab, Eur. J. Med. Chem., 2009, 44, 725; DOI: https://doi.org/10.1016/j.ejmech.2008.05.004.
A. Ahamed, I. A. Arif, M. Mateen, R. Surendra Kumar, A. Idhayadhulla, Saudi J. Biol. Sci., 2018, 25, 1227; DOI: https://doi.org/10.1016/j.sjbs.2018.03.001.
J. Lin, H. Deng, L. Jin, P. Pandey, J. Quinn, S. Cantin, J. E. Strickler, J. Med. Chem., 2006, 49, 7781; DOI: https://doi.org/10.1021/jm060978s.
M. D. Hall, N. K. Salam, J. L. Hellawell, H. M. Fales, C. B. Kensler, J. A. Ludwig, G. Szakács, D. E. Hibbs, M. M. Gottesman, J. Med. Chem., 2009, 52, 3191; DOI: https://doi.org/10.1021/jm800861c.
G. A. Gazieva, A. N. Kravchenko, Russ. Chem. Rev., 2012, 81, 494; DOI: https://doi.org/10.1070/rc2012v081n06abeh004235.
S. M. Mustafa, V. A. Nair, J. P. Chiffoor, S. Krishnapilla, Mini Rev. Org. Chem., 2004, 1, 375; DOI: https://doi.org/10.2174/1570193043403082.
S. Cascioferro, J. Med. Chem., 2020, 63, 7923; DOI: https://doi.org/10.1021/acs.jmedchem.9b01245.
W. M. Eldehna, J. Enzyme Inhib. Med. Chem., 2018, 33, 867; DOI: https://doi.org/10.1080/14756366.2018.1462802.
Ye. V. Leshcheva, Kh. S. Shikhaliev, G. V. Shatalov, G. I. Yermolova, Izv. vuzov. Khimiya i khim. tekhnologia [ChemChemTech], 2003, 46, 105 (in Russian).
T. I. El-Emary, R. A. Ahmed, E. A. Bakhite, J. Chin. Chem. Soc., 2001, 48, 921; DOI: https://doi.org/10.1002/jccs.200100134.
L. A. B. Freitas, A. C. S. Santos, G. C. Silva, F. N. N. Albuquerque, E. Silva, C. A. Simone, V. R. A. Pereira, L. Alves, F. Brayner, A. L. Leite, P. A. T. M. Gomes, Chem. Biol. Interact., 2021, 345, 109561; DOI: https://doi.org/10.1016/j.cbi.2021.109561.
A. M. Amani, Bulg. Chem. Commun., 2014, 46, 795.
S. Adhikari, S. B. Bari, A. Samanta, J. Appl. Chem. Res., 2014, 8, 31.
Z. Xie, G. Wang, J. Wang, M. Chen, Y. Peng, L. Li, W. Li, Molecules, 2017, 22, 659; DOI: https://doi.org/10.3390/molecules22040659.
R. Meleddu, S. Distinto, A. Corona, G. Bianco, V. Cannas, F. Esposito, A. Artese, S. Alcaro, P. Matyus, D. Bogdand, F. Cottiglia, E. Tramontano, E. Maccioni, Eur. J. Med. Chem., 2015, 93, 452; DOI: https://doi.org/10.1016/j.ejmech.2015.02.032.
M. L. Kondratieva, A. V. Pepeleva, N. P. Belskaia, A. V. Koksharov, P. V. Groundwater, K. Robeyns, L. Van Meervelt, W. Dehaen, Z.-J. Fan, V. A. Bakulev, Tetrahedron, 2007, 63, 3042; DOI: https://doi.org/10.1016/j.tet.2007.01.059.
E. A. Fayed, A. Ragab, R. R. E. Eldin, A. H. Bayoumi, Y. A. Ammar, Bioorg. Chem., 2021, 116, 105300; DOI: https://doi.org/10.1016/j.bioorg.2021.105300.
H. K. Mahmoud, T. A. Farghaly, H. G. Abdulwahab, N. T. Al-Qurashi, M. R. Shaaban, Eur. J. Med. Chem., 2020, 208, 112752; DOI: https://doi.org/10.1016/j.ejmech.2020.112752.
A. Yu. Potapov, B. V. Paponov, N. A. Podoplelova, M. A. Panteleev, V. A. Polikarchuk, I. V. Ledenyova, N. V. Stolpovskaya, D. V. Kryl’skii, Kh. S. Shikhaliev, Russ. Chem. Bull., 2021, 70, 492; DOI: https://doi.org/10.1007/s11172-021-3114-6.
GraphPad Prism, GraphPad, San Diego (CA), USA.
OriginPro 8, OriginLab Corp., Northampton (MA), USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the Russian Science Foundation (Project No. 18-74-10097, https://rscf.ru/project/21-74-03011/).
No human or animal subjects were used in this research.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 1969–1975, September, 2022.
Rights and permissions
About this article
Cite this article
Novichikhina, N.P., Ashrafova, Z.E., Stolpovskaya, N.V. et al. Synthesis and properties of novel hybrid molecules bearing 4H-pyrrolo[3,2,1-ij]quinolin-2-one and thiazole moieties. Russ Chem Bull 71, 1969–1975 (2022). https://doi.org/10.1007/s11172-022-3615-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3615-y