Abstract
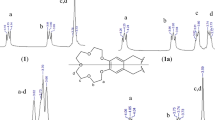
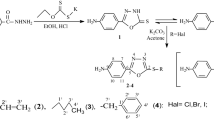
New Schiff bases were synthesized by condensation of 4-pyridinecarboxaldehyde with crown ethers (4-aminobenzo-15-crown-5, 4,4-diaminodibenzo-18-crown-6, and 4,4-diaminodibenzo-21-crown-7). Treatment of the synthesized azomethines with equimolar amount of copper(II) acetate gave the corresponding copper complexes. Antimicrobial activity of crown ethers and their derivatives against Gram-positive (Staphylococcus aureus, Micrococcus luteus) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa) bacteria was studied. The effects of the substituents on antimicrobial activity of the ligands and their derivatives were analyzed.
Similar content being viewed by others
References
C. Gumila, M. L. Ancelin, A. M. Delort, G. Jeminet, Antimicrob. Agents Chemother., 1997, 41, 523–529; DOI: https://doi.org/10.1128/AAC.41.3.523.
K. Yagi, V. Garcia, M. E. Rivas, J. Salas, A. Camargo, T. Tabata, J. Inclusion Phenom., 1984, 2, 179–184; DOI: https://doi.org/10.1007/BF00663254.
A. Gund, B. K. Keppler, J. Inorg. Biochem., 1993, 51, 437; DOI: https://doi.org/10.1016/0162-0134(93)85466-L.
N. Yu. Sadovskaya, B. N. Glushko, L. I. Blokhina, V. M. Retivov, Khim. Bezopasnost’ [Chem. Safety Sci.], 2020, 4, No. 2, 80; DOI: https://doi.org/10.25514/CHS.2020.2.18006 (in Russian).
M. Marjanović, M. Kralj, F. Supek, L. Frkanec, I. Piantanida, T. Šmuc, J. Med. Chem., 2007, 50, 1007; DOI: https://doi.org/10.1021/jm061162u.
G. W. Gokel, S. Negin, R. Cantwell, in Comprehensive Supramolecular Chemistry II, Ed. J. L. Atwood, Elsevier, Amsterdam, 2017, p. 3–48; DOI: https://doi.org/10.1016/B978-0-12-409547-2.12519-3.
M. Yildiz, A. Kiraz, B. Dülger, J. Serb. Chem. Soc., 2007, 72, 215; DOI: https://doi.org/10.2298/JSC0703215Y.
S. J. Franklin, Curr. Opin. Chem. Biol., 2001, 5, 201; DOI: https://doi.org/10.1016/s1367-5931(00)00191-5.
N. Yu. Sadovskaya, V. N. Glushko, M. A. Baryshnikova, D. A. Afanasyeva, M. Yu. Zhila, S. K. Belus, Russ. J. Gen. Chem., 2019, 89, 440; DOI: https://doi.org/10.1134/S1070363219030125.
M. Hiraoka, Crown Compounds: Their Characteristics and Applications, Elsevier, Amsterdam—New York, 1982, 275 pp.
H. Al-Amery, B. I. Al-Abdaly, M. K. Albayaty, Orient. J. Chem., 2016, 32, 1025; DOI: https://doi.org/10.13005/ojc/3202282.
M. Febles, S. Montalvao, G. D. Crespin, M. Norte, J. M. Padron, P. Tammela, J. J. Fernandez, A. H. Daranas, Bioorg. Med. Chem. Lett., 2016, 26, 5591; DOI: https://doi.org/10.1016/j.bmcl.2016.09.0662.
Z. Hayvali, H. Guler, H. Ogutcu, N. Sari, Med. Chem. Res., 2014, 23, 3652, DOI: https://doi.org/10.1007/s00044-014-0937-94.
M. B. Patel, E. Garrad, J. W. Meisel, S. Negin, M. R. Gokel, G. W. Gokel, RSC Adv., 2019, 9, 2217; DOI: https://doi.org/10.1039/c8ra07641c.
M. De Rosa, G. Vigliotta, A. Soriente, V. Capaccio, G. Gorrasi, R. Adami, E. Reverchon, M. Mella, L. Izzo, Biomater. Sci., 2017, 5, 741; DOI: https://doi.org/10.1039/c6bm00950f.
W. W. Tso, W.-P. Fung, M. Y. Tso, J. Inorg. Biochem., 1981, 14, 237; DOI: https://doi.org/10.1016/S0162-0134(00)80003-3.
W. W. Tso, W.-P. Fung, Inorg. Chim. Acta, 1980, 46, 33; DOI: https://doi.org/10.1016/S0020-1693(00)84130-4.
W. M. Leevy, M. E. Weber, M. R. Gokel, G. B. Hughes-Strange, D. D. Daranciang, R. Ferdani, G. W. Gokel, Org. Biomol. Chem., 2005, 9, 1647; DOI: https://doi.org/10.1039/B418194H.
L. A. Konup, I. P. Konup, V. E. Sklyar, K. N. Kosenko, V. P. Gorodnyuk, G. V. Fedorova, E. I. Nazarov, S. A. Kotlyar, Pharm. Chem. J., 1989, 23, 402; DOI: https://doi.org/10.1007/BF00758292.
H. I. Uğraş, U. Çakir, A. Azizoğlu, T. Kiliç, C. Erk, J. Inclusion Phenom. Macrocycl. Chem., 2006, 55, 159; DOI: https://doi.org/10.1007/s10847-005-9032-7.
V. N. Glushko, N. Yu. Sadovskaya, L. I. Blokhina, M. Yu. Zhila, S. K. Belus’, E. S. Vashchenkova, I. A. Shmeleva, Russ. J. Gen. Chem., 2018, 88, 1595; DOI: https://doi.org/10.1134/S1070363218080078.
Z. K. Khoramdareh, S. A. Hosseini-Yazdi, B. Spingler, A. A. Khandar, Inorg. Chim. Acta, 2014, 415, 7; DOI: https://doi.org/10.1016/j.ica.2014.02.022.
Funding
Analytical studies were performed with the use of the scientific equipment of the Center for Collective Use of National Research Centre “Kurchatov Institute” — IREA with the financial support from the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-11-2021-070 dated 19.08.2021).
Author information
Authors and Affiliations
Corresponding authors
Additional information
No human or animal subjects were used in this research.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1300–1304, June, 2022.
Rights and permissions
About this article
Cite this article
Sadovskaya, N.Y., Glushko, V.N., Blokhina, L.I. et al. Synthesis and studies of antimicrobial activity of azomethine crown ether derivatives and their copper complexes. Russ Chem Bull 71, 1300–1304 (2022). https://doi.org/10.1007/s11172-022-3534-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3534-y