Abstract
Imidazopyridine and pyranopyrazole derivatives include a very important class of heterocyclic compounds that have many biological properties and have been observed in the structure of many drugs and bioactive molecules. In this research project, we first successfully made copper catalyst immobilized on magnetic iron nanoparticles modified with dopamine and 1H-benzo[d]imidazole-2-carboxylic acid (as magnetic ligand) and confirmed its structure with a series of spectroscopic techniques; then in the next step, the catalytic activity of this nanocomposite [Fe3O4@Dop/Amide-BenzImid-CuBr2] was evaluated the nanocomposite in the multicomponent synthesis of imidazo[1,2-a]pyridine and pyranopyrazole derivatives. The results clearly showed that this magnetic nanocatalyst in water solvent under reflux conditions is a very efficient catalytic system for the synthesis of imidazo[1,2-a]pyridines and pyranopyrazoles because all the products were synthesized with high to excellent yields in a suitable period of time. Also, the recovery tests confirmed that the Fe3O4@Dop/Amide-BenzImid-CuBr2 catalyst can be reused up to 8 times without significantly reducing its catalytic efficiency. VSM and ICP-OES analyses confirmed the stability, magnetism and high efficiency of the catalyst after repeated use of the catalyst.
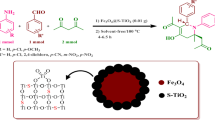
Graphical abstract





















Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
D. Bhattacherjee, M. Rahman, S. Ghosh, A.K. Bagdi, G.V. Zyryanov, O.N. Chupakhin, P. Das, A. Hajra, Adv. Synth. Catal. 363, 1597 (2021)
T. Wakaki, T. Togo, D. Yoshidome, Y. Kuninobu, M. Kanai, ACS Catal. 8, 3123 (2018)
J.A. Angulwar, G.S. Khansole, V.N. Bhosale, J. Synth. Chem. 1, 97 (2022)
M. Mohammadi, M. Khodamorady, B. Tahmasbi, K. Bahrami, A. Ghorbani-Choghamarani, J. Ind. Eng. Chem. 97, 1 (2021)
Y. Zhao, ACS Appl. Nano Mater. 3, 4917 (2020)
A. Arabmarkadeh, R. Javahershenas, M. Kazemi, Nanomaterials: Catalysis in synthesis of highly substituted heterocycles, Synth. Commun. 51, 880–903 (2021). https://doi.org/10.1080/00397911.2020.1864646
S. Ichie, H. Andre, Nanomater. Chem. 1, 32 (2023)
M.A.E.A.A.A. El-Remaily, Tetrahedron 70, 2971 (2014)
I.D. Inaloo, S. Majnooni, Eur. J. Org. Chem. 2019, 6359 (2019)
F. Ghobakhloo, M. Mohammadi, M. Ghaemi, D. Azarifar, Post-Synthetic Generation of Amino-Acid-Functionalized UiO-66-NH 2 Metal–Organic Framework Nanostructures as an Amphoteric Catalyst for Organic Reactions, ACS Appl. Nano Mater. 7, 1265–1277 (2024). https://doi.org/10.1021/acsanm.3c052
P. Moradi, M. Hajjami, New J. Chem. 45, 2981 (2021)
M. Mohammadi, A. Ghorbani-Choghamarani, S.M. Ramish, J. Mol. Struct. 1292, 136115 (2023)
S.A. Mousavi-Mashhadi, A. Shiri, ChemistrySelect 6, 3941 (2021)
M. Kazemi, M. Ghobadi, A. Mirzaie, Nanotechnol. Rev. 7, 43 (2018)
A.R. Sardarian, M. Zangiabadi, I.D. Inaloo, RSC Adv. 6, 92057 (2016)
A.R. Sardarian, I. DindarlooInaloo, M. Zangiabadi, New J. Chem. J. Chem. 43, 8557 (2019)
E. Bertolucci, A.M.R. Galletti, C. Antonetti, M. Marracci, B. Tellini, F. Piccinelli, C. Visone, in: 2015 IEEE International Instrumentation and Measurement Technology Conference Proceedings (IEEE, 2015), p. 1492.
W. Li, J. Yan, W. Xu, L.Y. Zhang, RSC Adv. 13, 28964 (2023)
M.A. Ashraf, Z. Liu, W.-X. Peng, L. Zhou, Catal. Lett. 150, 1128 (2020)
S. Gupta, J. Synth. Chem. 1, 37 (2022)
I. DindarlooInaloo, S. Majnooni, H. Eslahi, M. Esmaeilpour, Mol. Catal. 492, 110915 (2020)
S. Ghorbani, D. Habibi, S. Heydari, M. Mohammadi, M. Ariannezhad, Environ. Sci. Pollut. Res. 30, 32762 (2022)
A.N. Fajer, H.A. Al-Bahrani, A.A.H. Kadhum, M. Kazemi, J. Mol. Struct. 1296, 136800 (2024)
A.V. Nakhate, G.D. Yadav, ChemistrySelect 2, 7150 (2017)
N. Kazemi, M. Mahdavi Shahri, J. Inorg. Organomet. Polym. Mater. Inorg. Organomet. Polym. Mater. 27, 1264 (2017)
M. Kazemi, M. Mohammadi, Appl. Organomet. Chem. 34, e5400 (2020)
B. Pathare, T. Bansode, Results Chem. 3, 100200 (2021)
N. Rahman, G.S. Nongthombam, J.W.S. Rani, R. Nongrum, G.K. Kharmawlong, R. Nongkhlaw, Curr. Organocatal. 5, 150 (2018)
M. Kazemi, N. Karezani, Biol. Mol. Chem. 1, 15 (2023)
T. Palani, K. Park, M.R. Kumar, H.M. Jung, S. Lee, Eur. J. Org. Chem. 2012, 5038 (2012)
S. Mohana Roopan, S.M. Patil, J. Palaniraja, Res. Chem. Intermed. 42, 2749 (2016)
N. Devi, D. Singh, R.K. Rawal, J. Bariwal, V. Singh, Curr. Top. Med. Chem. 16, 2963 (2016)
A.J. Stasyuk, M. Banasiewicz, M.K. Cyrański, D.T. Gryko, J. Org. Chem. 77, 5552 (2012)
H. de Salles, T. da Silva, C. Radatz, R. Affeldt, E. Benvenutti, P. Schneider, Imidazo[1,2-a]pyridine A3-Coupling Catalyzed by a Cu/SiO2 Material, J. Braz. Chem. Soc. 30, 1825–1833 (2019). https://doi.org/10.21577/0103-5053.20190089
T.H. Al-Tel, R.A. Al-Qawasmeh, R. Zaarour, Eur. J. Med. Chem. 46, 1874 (2011)
S. Ulloora, R. Shabaraya, A.V. Adhikari, Bioorg. Med. Chem. Lett. 23, 3368 (2013)
A.K. Bagdi, S. Mitra, M. Ghosh, A. Hajra, Org. Biomol. Chem. 13, 3314 (2015)
R. Salim, E. Ech-chihbi, H. Oudda, F. El Hajjaji, M. Taleb, S. Jodeh, J. Bio-Tribo-Corros. 5, 14 (2019)
L.-R. Wen, Z.-R. Li, M. Li, H. Cao, Green Chem. 14, 707 (2012)
J. Panda, B.P. Raiguru, M. Mishra, S. Mohapatra, S. Nayak, Recent Advances in the Synthesis of Imidazo[1,2–a ]pyridines: A Brief Review, ChemistrySelect. 7, e202103987 (2022). https://doi.org/10.1002/slct.202103987
N. Nagarajan, G. Velmurugan, A. Prakash, N. Shakti, M. Katiyar, P. Venuvanalingam, R. Renganathan, Chem. Asian J. 9, 294 (2014)
E. Soleimani, M. Jafarzadeh, P. Norouzi, J. Dayou, C.S. Sipaut, R.F. Mansa, P. Saei, J. Chin. Chem. Soc. 62, 1155 (2015)
S.C. Jadhvar, H.M. Kasraliker, S.V. Goswami, A.V. Chakrawar, S.R. Bhusare, Res. Chem. Intermed. 43, 7211 (2017)
M. Kazemi, L. Shiri, H. Kohzadi, J. Mater. Environ. Sci. 8, 3410 (2017)
S. Karami, M.G. Dekamin, E. Valiey, P. Shakib, New J. Chem. 44, 13952 (2020)
Y. Liao, B. Huang, X. Huang, M. Cai, ChemistrySelect 4, 2320 (2019)
A. Noory Fajer, H. Khabt Aboud, H.A. Al-Bahrani, M. Kazemi, Recent Advances on Multicomponent Synthesis of Pyranopyrazoles Using Magnetically Recoverable Nanocatalysts, Polycycl. Aromat. Compd. 44, 1–47 (2024). https://doi.org/10.1080/10406638.2023.2255723
C. Zong, R. Zeng, J. Zou, Chem. Res. Chin. Univ. 30, 632 (2014)
J.B. Bharate, S.K. Guru, S.K. Jain, S. Meena, P.P. Singh, S. Bhushan, B. Singh, S.B. Bharate, R.A. Vishwakarma, RSC Adv. 3, 20869 (2013)
P. Shrivas, R. Pandey, S. Zodape, A. Wankhade, U. Pratap, Res. Chem. Intermed. 46, 2805 (2020)
H.T.H.D. Nguyen, M.-N.H.N.H. Truong, T. Van Le, N.T. Vo, H.T.H.D. Nguyen, P.H. Tran, ACS Omega 7, 17432 (2022)
D. Mallah, B.B.F. Mirjalili, BMC Chem. 17, 10 (2023)
Y. Chen, Z. Zhang, W. Jiang, M. Zhang, Y. Li, Mol. Divers. 23, 421 (2019)
Author information
Authors and Affiliations
Contributions
YL performed experimental works and analysis. XL performed experimental works. YS managed the project and wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Lin, X. & Sun, Y. Fabrication and characterization of copper complex immobilized on magnetic nanoparticles: an efficient and ecofriendly nanomagnetic catalyst for synthesis of imidazo[1,2-a]pyridines and pyranopyrazoles. Res Chem Intermed (2024). https://doi.org/10.1007/s11164-024-05264-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11164-024-05264-y