Abstract
The absolute necessity to fight some class of tumour is perceived as serious health concerns, and the discovery and development of effective anticancer agents are urgently needed. So, the novel ligands (N4MacL1–N6MacL3) and bivalent cobalt complexes with tetraaza macrocyclic ligands were produced and characterised using spectroscopic methods including physico-chemical and spectral (FTIR, UV–visible, 1H-NMR, 13C-NMR, Mass, EPR, TGA, molar-conductance measurements and PXRD) studies. The FTIR spectrum data point to the ligands' chelation behaviour towards Cobalt metal ion through the secondary amine group's nitrogen atom. The complexes' octahedral geometry was verified by the EPR spectrum data and UV–vis spectroscopy results. Then, molar conductance measurements confirmed the non-electrolytic nature of the complexes. Density theoretical calculations of the compounds' computational aspects have been examined and found to be in strong agreement with the outcomes of the experiments. Through simultaneous thermogravimetric analysis (TGA), thermal behaviour of the compounds was examined. Kinetic and thermodynamic parameters were calculated using Coats Redfern and Ozawa Fyn-wall method. The in vitro antimicrobial effectiveness of these resulting compounds has been scrutinized against a number of bacterial strains (E. coli, S. Marcescence, S. aureus) and fungal strains (A. flavus, C. albicans, and F. oxysporum) by disc diffusion method and prominent results were obtained. Complex [Co(N6MacL3)Cl2] has shown excellent antibacterial and antifungal activity with MIC 3.50 µM and 4.25 µM, respectively. Furthermore, the compounds have also been tested for antioxidant activity. The complexes [Co(N6MacL2)Cl2] and [Co(N6MacL3)Cl2] exhibited the upright activity as antioxidant. In addition, in vitro cytotoxic activity of the compounds was screened against human cervical cancer cells (HeLa), human breast cancer cells (MCF-7), IMR-32 and A549 using MTT assay. The complexes [Co(N6MacL2)Cl2] and [Co(N6MacL3)Cl2] showed the tremendous cytotoxic activity. For targeting molecular docking study, the drugs were tested using the receptors 5H67, 3TY7, 3DRA, 3ROW and 3T88.
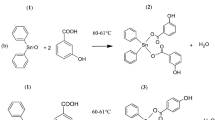
Graphical abstract


















Similar content being viewed by others
Data availability:
All data are available in the article and its supplementary material.
References
H. Hrichi, N.A. Elkanzi, A.M. Ali, A. Abdou, Res. Chem. Intermed. 49(5), 2257 (2023)
A. A. S. Al‐Hamdani, W. Al‐Zoubi, Spectrochim. Acta. Part A: Mole. and Biomol. Spect. 75, 137
M.A. Arafath, F. Adam, M.B.K. Ahamed, M.R. Karim, M.N. Uddin, B.M. Yamin, A. Abdou, J. Mol. Struct. 1278, 134887 (2023)
K. Babic-Samardžija, N. Hackerman, S.P. Sovilj, V.M. Jovanovic, J. Solid State Electrochem. 12, 155 (2008)
V.M. Jovanovic, K. Babic-Samardžija, S.P. Sovilj, Electroanal 13, 1129 (2001)
W. Sibert, A.H. Cory, J.G. Cory, Lipophilic derivatives of cyclam as new inhibitors of tumor cell growth, J. Chem. Soc. Chem. Commun. 2, 154 (2002)
S.J. Paisey, P.J. Sadler, Chem. Commun. 3, 306 (2004)
X. Liang, J.A. Parkinson, M. Weishaulp, R.O. Gould, S.J. Paisey, H. Park, T.M. Hunter, C.A. Blindauer, S. Parsons, P.J. Sadler, J. Am. Chem. Soc. 124, 9105 (2002)
C. Maxim, M. Badea, A.M. Rostas, M.C. Chifiriuc, G.G. Pircalabioru, S. Avram, R. Olar, Appl. Organomet. Chem. 36(1), e6471 (2022)
R.I. Maurer, P.J. Blower, J.R. Dilworth, C.A. Reynolds, Y. Zheng, G.E.D. Mullen, J. Med. Chem. 45, 1420 (2002)
A.R. Cowly, J.R. Dilworth, P.S. Donnely, E. Labisbal, A. Sousa, J. Am. Chem. Soc. 124, 5270 (2002)
M.B. Ferrari, F. Bisceglie, G. Pelosi, M. Sassi, P. Tarasconi, M. Cornia, S. Capacchi, R. Albertini, S. Pinelli, J. Inorg. Biochem. 90, 113 (2002)
E.M. Jouad, X.D. Thanh, G. Bouet, S. Bonneau, M.A. Khan, Anticancer Res 22, 1713 (2002)
M.E. Narayanan, R.A. Sosa-Torres, Toscano, J. Chem. Crystallogr. 31, 129 (2001)
J. Costamagna, G. Ferraudi, B. Matsuhiro, M. Campos-Vallete, J. Canales, M. Villagran, J. Vargas, M.J. Aguirre, Coord. Chem. Rev 196, 125 (2000)
L. Chaabane, H. Chahdoura, W. Moslah, M. Snoussi, E. Beyou, M. Lahcini, M.H.V. Baouab, Appl. Organometall. Chem. 33(5), e4860 (2019)
G. Vuckovi, S.B. Tanaskovic, U. Rychlewska, D.D. Radanovic, J. Mrozinski, M. Korabik, J. Mol. Struct. 827, 80 (2007)
E.M. Zayed, Egypt J Chem 65, 281 (2022)
T. Yang, H. Wu, S.-J. Ma, Y. Wang, Transitio. Met. Chem. 36, 403 (2011)
L. Bian, L. Li, Q. Zhang, J. Dong, T. Xu, J. Li, J. Kong, Transitio. Met. Chem. 37, 783 (2012)
R. Senthil Kumar, A. Riyasdeen, M. Dinesh, C. Preethy Paul, S. Srinag, H. Krishnamurthy, S. Arunachalam, M. Abdulkadher Akbarsha, (2011) Arch. Pharm. Chem. Life Sci. 344, 422
Z.H. Chohan, C.T. Supuran, J. Enz. Inhib. Med. Chem. 20(5), 463 (2005)
N.H. Mahmoud, G.H. Elsayed, A. Aboelnaga, A.M. Fahim, Appl. Organomet. Chem. 36(7), e6697 (2022)
A. Singh, H.P. Gogoi, P. Barman, A.K. Guha, Appl. Organomet. Chem. 36(6), e6673 (2022)
S. Osinsky, I. Levitin, L. Bubnovskaya, A. Sigan, I. Ganusevich, A. Kovelskaya, N. Valkovskaya, L. Campanella, P. Wardman, Exp. Oncol. 26, 140 (2004)
R. Senthil Kumar, S. Arunachalam, V.S. Periasamy, C.P. Preethy, A. Riyasdeen, M.A. Akbarsha, (2008), Polyhedron, 27, 1111–1120
D.P. Singh, V. Malik, R. Kumar, K. Kumar, K., J. Serb. Chem. Soc 75(6), 763 (2010)
Subhash, A. Chaudhary, Jyoti, M. Kumar, & R. Solanki, J. Iran. Chem. Soc., 20(7), 1–24, (2023)
N.A. Elkanzi, A.M. Ali, M. Albqmi, A. Abdou, Appl. Organomet. Chem. 36(11), e6868 (2022)
N.A. Elkanzi, H. Hrichi, H. Salah, M. Albqmi, A.M. Ali, A. Abdou, Polyhedron 230, 116219 (2023)
P. Bomzan, N. Roy, B. Ghosh, M.N. Roy, J. Mol. Struct. 1271, 133981 (2023)
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. Warren, H. Bokesch, S. Kenney, M. Boyd, J. Natl. Cancer Inst. 82, 1107 (1990)
A. Monks, D. Scudiero, P. Skehan, R. Shoemaker, K. Paull, D. Vistica, C. Hose, J. Langley, P. Cronise, A. Vaigro-wolff et al., J. Natl. Cancer Inst. 83, 757 (1991)
J. Carmichael, W.G. Degraff, A.F. Gazdar, J.D. Minna, J.B. Mitchell, Am. Assoc. Cancer Res. 47, 936 (1987)
A. Abdou, H.M. Mostafa, A.M.M. Abdel-Mawgoud, Inorg. Chim. Acta 539, 121043 (2022)
A. Braca, N. De Tommasi, L. Di Bari, C. Pizza, M. Politi, I. Morelli, J. Nat. Prod. 64, 892 (2001)
G.A. Giffin, A. Moretti, S. Jeong, S. Passerini, J. Phys. Chem. C 118, 9966 (2014)
L. Kathawate, P.V. Joshi, T.K. Dash, S. Pal, M. Nikalje, T. Weyhermüller, S. Salunke-Gawali, S. J. Mol. Struct. 1075, 397 (2014)
A.A. Osowole, A.C. Ekennia, O.O. Olubiyi, M. Olagunju, Res. Chem. Intermed. 43, 2555 (2016)
N. Feizi, R.V. Pinjari, S. Gejji, F. Sayyed, R. Gonnade, S.Y. Rane, J. Mol. Struct. 966, 144 (2010)
S. Jyothi, K. Sreedhar, D. Nagavaju, S.J. Swamy, Can Chem Trans. 3, 368 (2015)
M. Tyagi, S. Chandra, Open. J. Inorg. Chem. 2, 41 (2012)
S.J. Swamy, P. Someshwar, Spectrochim. Acta a 70, 929 (2008)
A.A. Osowole, S.A. Balogun, Eur. J. Appl. Sci. 4, 6 (2012)
A. C/ Ekennia, A. A. Osowole, D. C. Onwudiwe, I. Babahan, C. U. Ibeji, S. N. Okafor, & O. T. Ujam, (2018) Applied Organometallic Chemistry, 32(5), e4310
S.M. Abdallah, G.G. Mohamed, M.A. Zayed, M.S.A. El-Ela, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 73, 833 (2009)
R. Kumar, S. Obrai, A.K. Jassal, M.S. Hundal, J. Mitra, S. Sharma, J. Coord. Chem. 68, 2130 (2015)
A. Hudak, A. Kosturiak, J. Therm. Anal. Calori. 58, 579 (1999)
S. Materazzi, G.D. Ascenzo, S. Aquili, K.M. Kadish, J.L. Bear, Thermochim. Acta 397, 129 (2003)
O.A. El-Gammal, G.A. El-Reash, R.A. Bedier, Appl. Organomet. Chem. 33(10), e5141 (2019)
V. Pushpanathan, S.S.J. Dhas, D.S. Kumar, Bull. Mater. Sci. 44, 1 (2021)
Y. A. Alghuwainem, H. M. Abd El-Lateef, M. M. Khalaf, A. A. Abdelhamid, A. Alfarsi, M. Gouda, & A. Abdou, J. Mol. Liq., 369, 120936, (2023)
M. Kumar, P.J. Darolia, S. Chauhan, M. Sindhu, K.K. Verma, S. Garg, ChemistrySelect 6(23), 5778 (2021)
M. A. I. Al-Gaber, H. M. Abd El-Lateef, M. M. Khalaf, S. Shaaban, M. Shawky, G. G. Mohamed, & A. M. Abu-Dief, Materials, 16(3), 897, (2023)
M.S. Hossain, K.A. Khushy, M.A. Latif, M.F. Hossen, M.A. Asraf, M. Kudrat-E-Zahan, A. Abdou, Russ. J. Gen. Chem. 92(12), 2723 (2022)
M.A. Latif, T. Ahmed, M.S. Hossain, B.M. Chaki, A. Abdou, M. Kudrat-E-Zahan, Russ. J. Gen. Chem. 93(2), 389 (2023)
M. A. I. Al-Gaber, H. M. Abd El-Lateef, M. M. Khalaf, S. Shaaban, M. Shawky, G. G. Mohamed, & A. M. Abu-Dief, Materials, 16(3), 897, (2023).
B.V. Kumar, H.B. Naik, D. Girija, N. Sharath, S.M. Pradeepa, H.J. Hoskeri, M.C. Prabhakara, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 94, 192 (2012)
J. Haribabu, G.R. Subhashree, S. Saranya, K. Gomathi, R. Karvembu, D. Gayathri, J. Mol. Struct. 1094, 281 (2015)
A. J. Jarad, M. A. Dahi, T. H. Al-Noor, M. M. El-ajaily, S. R. AL-Ayash, & A. Abdou, A. Journal of Molecular Structure, 1287, 135703, (2023)
Subhash, A. Chaudhary, Jyoti, M. Kumar, N. Kumar, & N. K. Agarwal, Journal of Chemical Sciences, 134(4), 113, (2022)
Y.A. Alghuwainem, H.M.A. El-Lateef, M.M. Khalaf, A.A. Amer, A.A. Abdelhamid, A.A. Alzharani, A. Abdou, J Mol. Sci. 23(24), 15614 (2022)
Acknowledgements
One of the authors Subhash is grateful to University Grants Commission (UGC), New Delhi, for financial assistance in the form of SRF, (Ref. No.- 92(CSIR-UGC NET DEC. 2018). The authors are grateful to DST-FIST Program 2017 (Final proposal no. SR/FST/CS-J/2017/12(c) dated 10.05.2018, Department of Chemistry, Kurukshetra university, Kurukshetra.
Funding
The Funding was provided by University Grants Commission, 92(CSIR-UGC NET DEC. 2018)
Author information
Authors and Affiliations
Contributions
SUBHASH contributed to conceptualization, data curation, formal analysis, funding acquisition, methodology, software, writing—original draft, and writing—review and editing. JYOTI contributed to conceptualization, data curation, formal analysis, methodology, software, writing—original draft, and writing—review and editing. ASHU CHAUDHARY contributed to conceptualization, data curation, formal analysis, methodology, supervision, and visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Subhash, Jyoti & Chaudhary, A. Synthesis, spectroscopic characterization, in vitro cytotoxic, antimicrobial and antioxidant studies of Co(II) complexes bearing pyridine-based macrocyclic ligands with density function theory (DFT) and molecular docking investigations. Res Chem Intermed 49, 4729–4758 (2023). https://doi.org/10.1007/s11164-023-05096-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05096-2