Abstract
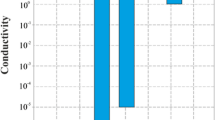
Polymer materials have been widely studied due to their large potential of application in technological fields because of their excellent and diverse properties. Morphological and rearrangement state of synthesized polymers has significant effect on their physicochemical properties and influence the performance of these materials in thin films structures. It is of both fundamental and practical importance to understand the effect of synthesis parameters and changing such parameters on optical, electronic, electrical and morphological properties of polymer surfaces and thin films for the development of new functional polymers and their applications. In this article, the fundamentals of optical, charge career and morphological mechanics are presented for quantifying the use of synthesis parameters for thin films physicochemical properties tuning. Pyrrole with 2-nitrocinnamaldehyde based copolymers have been synthetized by varying synthesis parameters such solvent, reaction time and temperature which proved a relevant effect on the morphological and optoelectronic properties of the relative final deposited thin films. The study proved that the best opto-electric properties are obtained by using either acetone with a long reaction time or chloroform with a short reaction time and room conditions for the pressure and reaction temperature. The optimized PPNC thin films showed a narrow absorbance covering the entire visible optical spectrum and a considerable conductivity of 10.3 104 S/cm ensured by a charge mobility of 677.1 cm2/Vs. The electrochemical study proved that the electronic energy level could be optimized from the synthesis parameters as well as the gap varying around 3.0 eV depending on the optimal conditions.








Similar content being viewed by others
Data availability
Not applicable.
References
S. Tokonami, Y. Nakadoi, H. Nakata, S. Takami, T. Kadoma, H. Shiigi, T. Nagaoka, Res. Chem. Intermed. 40, 2327 (2014)
N. Ohta, T. Tanaka, I. Yamazaki, Res. Chem. Intermed. 27, 61 (2001)
P. Müller-Buschbaum, M. Stamm, Colloid Polym. Sci. 279, 376 (2001)
M.G. Faraj, P. Taboada, Inorg. Organomet. Polym. 27, 1405 (2017)
F. Mohanty, S.K. Swain, Polym. Compos. 40, 1199 (2019)
S. Ramesh, O. Uma, R. Shanti, L.J. Yi, K. Ramesh, Measurement 48, 263 (2014)
Y. Sun, B. Yang, G. Guo, L. Yaqing, Z. Guizhe, Inorg. Organomet. Polym. 21, 395 (2011)
G. Bidan, S. Guillerez, V. Sorokin, Adv. Mater. 8, 157 (1996)
L. Pu, Acta. Polym. 48, 116 (1997)
A. Patil, S. Shinde, G. Rashinkar, Res. Chem. Intermed. 46, 63 (2020)
J. Han, Adv. Opt. Mater. 6, 1800538 (2018)
O. Folorunso, Y. Hamam, R. Sadiku, S.S. Ray, G.J. Adekoya, FlatChem 26, 100211 (2021)
B. El-Hachemi, S. Miloud, M. Sabah, S. Touahri, Z. Ouili, B. Boudine, M.T. Soltani, O. Halimi, Inorg. Organomet. Polym. 31, 3637 (2021)
G. Sarojini, S. Venkateshbabu, M. Rajasimman, Chemosphere 278, 130400 (2021)
A.L. Pang, A. Arsad, M. Ahmadipour, Polym. Adv. Technol. 32, 1428 (2021)
W. Wang, H. Xu, W. Zhao, J. Zhao, M. Jiang, S. Liu, W. Huang, Q. Zhao, Chem. Eng. 428, 131089 (2022)
F. Ji, X. Guo, A. Liu, P. Xu, Y. Tan, R. Wang, L. Hao, Carbo. Polym. 270, 118362 (2021)
E.G.C. Ergun, B.B. Carbas, Mater. Today Commun. 302, 103888 (2022)
E. Martínez-Cartagena, J. Bernal-Martínez, C.A. Aranda-Sánchez, A. Banda-Villanuev, O.L. Gonzalez-Zapata, A. Ledezma-Pérez, A. Aguilar-Elguezabal, J. Romero-García, Mater. Sci. Chem. Eng. 9, 19 (2021)
R. Vaquero-Bermejo, E. Blázquez-Blázquez, M. Hoyos, J.M. Gómez-Elvira, Mater. Today Commun. 31, 103469 (2022)
C.H. Choi, M. Kertesz, J. Phys. Chem. A 101, 3823 (1997)
D. Lin-Vien, N.B. Colthup, W.G. Fateley, J.G. Grasselli, The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules (Academic, Boston, 1991)
Y. Gao, B. Cole, W.E. Tenhaeff, Macromol. Mater. Eng. 2, 1700425 (2017)
A.L. Mercado, C.E. Allmond, J.G. Hoekstra, J.M. Fitz-Gerald, Appl. Phys. A 81, 591 (2005)
I. Petrik, E. Frolova, A. Turchin, N. Smirnova, A. Eremenko, Res. Chem. Intermed. 45, 4113 (2019)
M. Koenig, V. Trouillet, A. Welle, K. Hinrichs, J. Lahann, Macromol. Chem. Phys. 19, 221 (2020)
S. Al-Bati, H. Aljboor, K. Ibtehaj, P.C. Ooi, B.A. Al-Asbahi, A.A.A. Ahmed, M.H.H. Jumali, ECS J. Solid State Sci. Technol. 11(5), 056002 (2022)
A. Remil, Y. Mouchaal, A. B. Reguig, A. Lakhdar Toumi, H. Gherrass, A. Hachemaoui , A. Yahiaoui, A. Khelil, Surf. Rev. Let. 25, 1850116 (2017)
Y. Mouchaal, H. Gherrass, A.B. Reguig, A. Hachemaoui, A. Yahiaoui, M. Makha, A. Khelil, J.C. Bernede, Eur. Phys. J. Appl. Phys. 69, 20203 (2015)
C.J. Mathai, S. Saravanan, M.R. Anantharaman, S. Venkitachalam, S. Jayalekshmi, J. Phys. D Appl. Phys. 35(17), 2206 (2002)
A. Nicosia, F. Vento, G.M. Di Mari, L. D’Urso, P.G. Mineo, Nanomaterials 11, 400 (2021)
K.H. Tan, L. Samylingam, N. Aslfattahi, R. Saidur, K. Kadirgama, Opt. Laser. Tech. 136, 106772 (2021)
G. Prunet, F. Pawula, G. Fleury, E. Cloutet, A.J. Robinson, G. Hadziioannou, A. Pakdel, Mater. Today Phys. 18, 100402 (2021)
W. Li, S. Zeiske, O.J. Sandberg, D.B. Riley, P. Meredith, A. Armin, Energy Environ. Sci. 14, 6484 (2021)
M.Y. Mehboob, M. Adnan, R. Hussain, F. Arshad, I. Zobia, Opt. Quant. Electron. 53, 517 (2021)
A. Kaewprajak, P. Kumnorkaew, K. Lohawet, B. Duong, T. Chonsut, N. Kayunkid, N. Saranrom, V. Promarak, Surf. Interf. 23, 100969 (2021)
F. Zhang, E. Mohammadi, X. Luo, J. Strzalka, J. Mei, Y. Diao, Langmuir 34, 1109 (2018)
D.M. Leeuw, M.M.J. Simenon, A.R. Brown, R.E.F. Einerhand, Synth. Met. 87, 53 (1997)
C.J. Yang, S.A. Jenekhe, Macromolecules 28, 1180 (1995)
A.K. Agrawal, S.A. Jenekhe, Chem. Mater 8, 579 (1996)
Acknowledgements
Authors gratefully acknowledge support by the funding granted by the Algerian Ministry of Higher Education and Scientific Research (MESRS) as well as the General Direction of Scientific Research and Scientific Development (DGRSDT). Author’s acknowledgements are presented to LCOMM team, University Mustapha Stambouli of Mascara for support on electrochemical analysis. Dr. Y. MOUCHAAL acknowledges the financial support from LPCMME lab team, Oran1 Ahmed Benbella University.
Funding
This work was supported by The Algerian Ministry of Higher Education and Scientific Research (MESRS) and General Direction of Scientific Research and Scientific Development (DGRSDT).
Author information
Authors and Affiliations
Contributions
YM and AA performed experiments and calculations. AH, ALAT, and AT contributed for DATA plot and analysis investigation. AA, YM, and AY wrote and performed the main manuscript. AK, YM, and AY supervised the work. All authors read and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anab, A., Mouchaal, Y., Yahiaoui, A. et al. Polymerization parameters diapason as a tool for thin films morphological and optoelectrical properties optimization of Poly (pyrrole-co-2-nitrocinnamaldehyde) conjugated semiconductor. Res Chem Intermed 49, 2141–2153 (2023). https://doi.org/10.1007/s11164-023-04970-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-04970-3