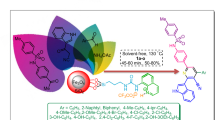
Abstract
A new pseudopolymeric nanomagnetic catalyst containing urea linkers, namely Fe3O4@SiO2@[(CH2)3–urea–(CH2)2–O–(CH2)]2, was synthesized. The mentioned nanomagnetic catalyst was characterized by using several methods including Fourier transform infrared spectroscopy, thermogravimetric/differential thermogravimetric analysis, vibrating sample magnetometer analysis, scanning electron microscopy, transmission electron microscopy, and energy-dispersive X‐ray analysis. In this research, we examined the catalytic usage of Fe3O4@SiO2@[(CH2)3–urea–(CH2)2–O–(CH2)]2 for the preparation of a wide range of 2‐amino‐3‐cyano and 2‐hydroxy‐3‐cyanopyridine derivatives bearing thiophene or furan moieties. All desired hybrid pyridines were synthesized in short reaction times with high yields. The crucial finding is that the catalyst was very efficient, stable, and easy retrieve. In addition, a vinylogous anomeric-based oxidation pathway is suggested as a mechanism for the preparation of target molecules.
Graphical abstract














Similar content being viewed by others
References
B. Changmai, G. Pathak, J.M.H. Anal, L. Rokhum, Mini Rev. Org. Chem. 17, 740 (2020)
A. Mukhtar, S. Saqib, H. Lin, M.U.H. Shah, S. Ullah, M. Younas, M. Rezakazemi, M. Ibrahim, A. Mahmood, S. Asif, Renew. Sustain. Energy Rev. 157, 112012 (2022)
L. Liu, A. Corma, Chem. Rev. 118, 4981 (2018)
M.F.I. Al-Hussein, M.S.S. Adam, Appl. Organomet. Chem. 34, 6 (2020)
M.S.S. Adam, M.A. Al-Omair, Appl. Organomet. Chem. 34, 12 (2020)
B. Karimi, F. Mansouri, H.M. Mirzaei, ChemCatChem 7, 1736 (2015)
M. Mokhtary, J. Iran. Chem. Soc. 13, 1827 (2016)
M. Miceli, P. Frontera, A. Macario, A. Malara, Catalysts 11, 591 (2021)
A. Sápi, T. Rajkumar, J. Kiss, Á. Kukovecz, Z. Kónya, G.A. Somorjai, Catal. Lett. 151, 2153 (2021)
M.B. Gawande, A. Goswami, T. Asefa, H. Guo, A.V. Biradar, D.L. Peng, R. Zboril, R.S. Varma, Chem. Soc. Rev. 44, 21 (2015)
S. Kezic, A. Kammeyer, F. Calkoen, J. Fluhr, J. Bos, Br. J. Dermatol. 161, 1098 (2009)
L. Celleno, Dermatol. Ther. 31, 12690 (2018)
H. Koizumi, K. Takeuchi, K. Matsumoto, N. Fukaya, K. Sato, M. Uchida, S. Matsumoto, S. Hamura, J.-C. Choi, Commun. Chem. 4, 66 (2021)
P.Y. Lam, P.K. Jadhav, C.J. Eyermann, C.N. Hodge, Y. Ru, L.T. Bacheler, J.L. Meek, M.J. Otto, M.M. Rayner, Y.N. Wong, Science 263, 380 (1994)
H.-Q. Li, P.-C. Lv, T. Yan, H.-L. Zhu, Anti Cancer Agents Med. Chem. 9, 471 (2009)
Market Research Center, in Global and Chinese Ethylene Urea Industry, Market Research Report (Prof Research, 2018).
B. Atashkar, M.A. Zolfigol, S. Mallakpour, J. Mol. Catal. 452, 192 (2018)
M.C. Gimeno, R.P. Herrera, Eur. J. Org 2020, 1056 (2020)
A. Skrzynska, S. Frankowski, L. Albrecht, Asian J. Org. Chem. 9, 1688 (2020)
M. Yokoya, S. Kimura, M. Yamanaka, Chem. Eur. J. 27, 5601 (2021)
C. Cimarelli, Molecules 24, 2372 (2019)
M.G. Dekamin, M. Azimoshan, L. Ramezani, Green Chem. 15, 811 (2013)
N. Isambert, M.D.M.S. Duque, J.-C. Plaquevent, Y. Genisson, J. Rodriguez, T. Constantieux, Chem. Soc. Rev. 40, 1347 (2011)
R. Kakuchi, Polym. J. 51, 945 (2019)
M.A. Meier, C. Barner-Kowollik, Adv. Mater. 31, 1806027 (2019)
A. Akbari, M.G. Dekamin, A. Yaghoubi, M.R. Naimi-Jamal, Sci. Rep. 10, 1 (2020)
A.C. Boukis, K. Reiter, M. Frölich, D. Hofheinz, M.A. Meier, Nat. Commun. 9, 1 (2018)
M. Torabi, M. Yarie, S. Baghery, M.A. Zolfigol, Recent Developments in the Synthesis and Applications of Pyridines, 503 (2023), ISBN: 978-0-323-91221–1.
F.M. Arlan, A.P. Marjani, R. Javahershenas, J. Khalafy, New J. Chem. 45, 12328 (2021)
S. Abdel-Raheem, A. El-Dean, R. Hassanien, M. El-Sayed, M. Sayed, A. Abd-Ella, Curr. Chem. Lett. 10, 255 (2021)
R.E. Khidre, I.A.M. Radini, Sci. Rep. 11, 7846 (2021)
C. Liu, L. Shi, J. Zhang, J. Sun, J. Chem. Eng. 427, 131633 (2022)
B. Rong, G. Xu, H. Yan, S. Zhang, Q. Wu, N. Zhu, Z. Fang, J. Duan, K. Guo, Org. Chem. Front. 8, 2939 (2021)
A. Negi, S.I. Mirallai, S. Konda, P.V. Murphy, Tetrahedron, 121, 132930 (2022)
D.A. Shabalin, Org. Biomol. Chem. 19, 8184 (2021)
S. Nanjundaswamy, S. Bindhu, R.R. Arun Renganathan, S. Nagashree, C.S. Karthik, P. Mallu, V. Ravishankar Rai, J. Biomol. Struct. Dyn. 1 (2021)
A. Yeşildağ, Chem. Pap. 75, 4949 (2021)
M.A. Abu-Zaied, G.H. Elgemeie, F.T. Halaweish, S.F. Hammad, Nucleosides Nucleotides Nucleic Acids, 41, 851 (2022)
S.H. Gebre, Synth. Commun. 51, 1669 (2021)
A.M.K. El-Dean, A.A. Abd-Ella, R. Hassanien, M.E. El-Sayed, S.A.A. Abdel-Raheem, ACS Omega 4, 8406 (2019)
V.I. Savchenko, V.G. Dorokhov, I.K. Yakushchenko, I.N. Zyuzin, S.M. Aldoshin, Her. Russ. Acad. Sci. 80, 149 (2010)
E. Nassar, J. Am. Sci. 6, 463 (2010)
R.M. Zaki, A.M. Kamal El-Dean, J.A. Mickey, N.A. Marzouk, R.H. Ahmed, Synth. Commun. 47, 2406 (2017)
E.A. Bakhite, A.A. Abd-Ella, M.E. El-Sayed, S.A. Abdel-Raheem, J. Agric. Food Chem. 62, 9982 (2014)
E.A. Bakhite, A.A. Abd-Ella, M.E. El-Sayed, S.A. Abdel-Raheem, J. Saudi Chem. Soc. 21, 95 (2017)
S.T. Chung, W.H. Huang, C.K. Huang, F.C. Liu, R.Y. Huang, C.C. Wu, A.R. Lee, Res. Chem. Intermed. 42, 1195 (2016)
S. Asadbegi, M.A. Bodaghifard, A. Mobinikhaledi, Res. Chem. Intermed. 46, 1629 (2020)
E.S. Ibrahim, G.E.H. Elgemeie, M.M. Abbasi, Y.A. Abbas, M.A. Elbadawi, A.M.E. Attia, Nucl. Nucleot. Nucl. Acids 14, 1415 (1995)
L. Prakash, S. Verma, E. Tyagi, R. Mital, J. Fluor. Chem. 41, 303 (1988)
T. Murata, M. Shimada, S. Sakakibara, T. Yoshino, H. Kadono, T. Masuda, M. Shimazaki, T. Shintani, K. Fuchikami, K. Sakai, Bioorg. Med. Chem. Lett. 13, 913 (2003)
M. Mantri, O. de Graaf, J. van Veldhoven, A. Göblyös, J.K. von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. Link, H. de Vries, M.W. Beukers and J. Brussee, J. Med. Chem. 51, 4449 (2008)
A. Thirupathi, M. Janni, S. Peruncheralathan, J. Org. Chem. 83, 8668 (2018)
G.M. Ziarani, Z. Kheilkordi, F. Mohajer, A. Badiei, R. Luque, RSC Adv. 11, 17456 (2021)
J.C. Xiang, M. Wang, Y. Cheng, A.-X. Wu, Org. Lett. 18, 24 (2016)
M. Muthu, R.V. Priya, A.I. Almansour, R.S. Kumar, R.R. Kumar Beilstein, J. Org. Chem. 14, 2907 (2018)
F. Tamaddon, D. Azadi, J. Mol. Liq. 249, 789 (2018)
A.A. Dissanayake, R.J. Staples, A.L. Odom, Adv. Synth. Catal. 356, 1811 (2014)
W.P. Griffith, T.Y. Koh, D.J. Williams, J. Chem. Soc. Dalton trans. 23, 3456 (1993)
H. Masui, A. Lever, P.R. Auburn, Inorg. Chem. 30, 2402 (1991)
C. Flouzat, Y. Bresson, A. Mattio, J. Bonnet, G. Guillaumet, J. Med. Chem. 36, 497 (1993)
S. Mostafa, A. El-Maksoud, Monatsh. Chem. 129, 455 (1998)
L. Forlani, G. Cristoni, C. Boga, P.E. Todesco, E. Del Vecchio, S. Selva, M. Monari, ARKIVOC 11, 198 (2002)
H.W. Yang, B.M. Craven, Acta Crystallogr. B: Struct. Sci. 54, 912 (1998)
I.V. Alabugin, L. Kuhn, M.G. Medvedev, N.V. Krivoshchapov, V.A. Vil, I.A. Yaremenko, P. Mehaffy, M. Yarie, A.O. Terentev, M.A. Zolfigol, Chem. Soc. Rev. 50, 10253 (2021)
I.V. Alabugin, L. Kuhn, N.V. Krivoshchapov, P. Mehaffy, M.G. Medvedev, Chem. Soc. Rev. 50, 10212 (2021)
M. Torabi, M. Yarie, M.A. Zolfigol, S. Azizian, Y. Gu, RSC Adv. 12, 8804 (2022)
F. Karimi, M. Yarie, M.A. Zolfigol, Mol. Catal. 497, 111201 (2020)
P. Ghasemi, M. Yarie, M.A. Zolfigol, A. Taherpour, M. Torabi, ACS Omega 5, 3207 (2020)
F. Karimi, M. Yarie, M.A. Zolfigol, RSC Adv. 10, 25828 (2020)
M. Dashteh, M.A. Zolfigol, A. Khazaei, S. Baghery, M. Yarie, S. Makhdoomi, M. Safaiee, RSC Adv. 10, 2782 (2020)
F. Karimi, M. Yarie, M.A. Zolfigol, Mol. Catal. 463, 20 (2019)
M. Torabi, M. Yarie, M.A. Zolfigol, S. Rouhani, S. Azizi, T.O. Olomola, M. Maaza, T.A.M. Msagati, RSC Adv. 11, 3143 (2020)
M. Yarie, Iran. J. Catal. 7, 85 (2017)
M. Yarie, Iran. J. Catal. 10, 79 (2020)
J. Afsar, M.A. Zolfigol, A. Khazaei, M. Zarei, Y. Gu, D.A. Alonso, A. Khoshnood, Mol. Catal. 482, 110666 (2020)
S. Babaee, M. Zarei, H. Sepehrmansourie, M.A. Zolfigol, S. Rostamnia, ACS Omega 5, 6240 (2020)
F. Jalili, M. Zarei, M.A. Zolfigol, S. Rostamnia, A.R. Moosavi-Zare, Microporous Mesoporous Mater. 294, 109865 (2020)
S. Kalhor, M. Yarie, M. Torabi, M.A. Zolfigol, M. Rezaeivala, Y. Gu, Polycycl. Aromat. Compd. 11, 17456 (2021)
M. Torabi, M.A. Zolfigol, M. Yarie, B. Notash, S. Azizian, M.M. Azandaryani, Sci. Rep. 11, 16846 (2021)
S. Kalhor, M. Zarei, M.A. Zolfigol, H. Sepehrmansourie, D. Nematollahi, S. Alizadeh, H. Shi, J. Arjomandi, Sci. Rep. 11, 19370 (2021)
M. Torabi, M. Yarie, F. Karimi, M.A. Zolfigol, Res. Chem. Intermed. 46, 5361 (2020)
K.R. Abdellatif, E.K. Abdelall, W.A. Fadaly, G.M. Kamel, Med. Chem. Res. 24, 2632 (2015)
P.V. Oliferenko, A.A. Oliferenko, A.S. Girgis, D.O. Saleh, A.M. Srour, R.F. George, G.G. Pillai, C.S. Panda, C.D. Hall, A.R. Katritzky, J. Chem. Inf. Model. 54, 1103 (2014)
A.A. El-Shehawy, M.E. Abdu, M.M. El-Hendawy, M. El-Khouly, M.H. Sherif, H.Y. Moustafa, J. Phys. Org. Chem. 34, 4158 (2021)
H.A. El-Sayed, A.H. Moustafa, A.E.-F.Z. Haikal, R. Abu-El-Halawa, H. El Sayed, Eur. J. Med. Chem. 46, 2948 (2011)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tavassoli, A.M., Zolfigol, M.A. & Yarie, M. Application of new multi-H-bond catalyst for the preparation of substituted pyridines via a cooperative vinylogous anomeric-based oxidation. Res Chem Intermed 49, 679–699 (2023). https://doi.org/10.1007/s11164-022-04875-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04875-7