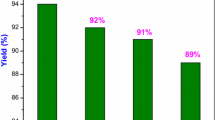
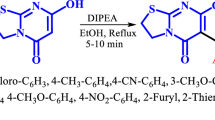
Abstract
The catalytic potential of organocatalyst N-methylimidazole is explored in the multicomponent reactions of aromatic aldehydes, malononitrile and 2,3-dihydro-phthalazine-1,4-dione/5-amino-2,3-dihydro-phthalazine-1,4-dione for the synthesis of pyrazolo[1,2-b]phthalazine-diones in ethanol under reflux conditions. The protocol has been successfully extended for the synthesis of pyrazolophthalazinyl quinolines using one-pot three-component reactions of 2-chloro-3-formylquinoline, malononitrile/ethyl cyanoacetate and 2,3-dihydro-phthalazine-1,4-dione/5-nitro-2,3-dihydro-phthalazine-1,4-dione. The reported method describes a straightforward and highly efficient protocol with remarkable advantages, including wide substrate scope, ready availability, lower cost of catalyst, operational simplicity and good to high isolated yields along with in-flask catalyst recycling.




Similar content being viewed by others
References
B. List, Chem. Rev. 107, 5413 (2007)
A. Dondoni, A. Massi, Angew. Chem. Int. Ed. 47, 4638 (2008)
G. Greco, Synlett 17, 2595 (2011)
A. Pande, K. Ganesan, A.K. Jain, P.K. Gupta, R.C. Malhotra, Org. Process Res. Dev. 9, 133 (2005)
H. Hagiwara, H. Inoguchi, M. Fukushima, T. Hoshi, T. Suzuki, Tetrahedron Lett. 47, 5371 (2006)
X.Z. Lian, Y. Huang, Y.Q. Li, W.J. Zheng, Monatsh. Chem. 139, 129 (2008)
A. Domling, Chem. Rev. 106, 17 (2006)
H.S. Oboudatian, J. Safaei-Ghomi, Sci. Rep. 12, 1 (2022)
H.S. Oboudatian, J. Safaei-Ghomi, Res. Chem. Intermed. 48, 2069 (2022)
N.K. Terrett, A.S. Bell, D. Brown, P. Ellis, Bioorg. Med. Chem. Lett. 6, 1819 (1996)
M.J. Genin, C. Biles, B.J. Keiser, S.M. Poppe, S.M. Swaney, W.G. Tarpley, Y. Yagi, D.L. Romero, J. Med. Chem. 43, 1034 (2000)
D. O’Hagan, J. Fluor. Chem. 131, 1071 (2010)
F. Al'-Assar, K.N. Zelenin, E.E. Lesiovskaya, I.P. Bezhan, B.A. Chakchir, Pharm. Chem. J. 36, 598 (2002)
R.P. Jain, J.C. Vederas, Bioorg. Med. Chem. Lett. 14, 3655 (2004)
S. Grasso, G. De Sarro, A. De Sarro, N. Micale, M. Zappala, G. Puja, M. Baraldi, C. De Micheli, J. Med. Chem. 43, 2851 (2000)
N. Watanabe, Y. Kabasawa, Y. Takase, M. Matsukura, K. Miyazaki, H. Ishihara, K. Kodama, H. Adachi, J. Med. Chem. 41, 3367 (1998)
A.M. Khalil, M.A. Berghot, M.A. Gouda, Eur J Med Chem. 44, 4448 (2009)
J.S. Kim, H.-J. Lee, M.-E. Suh, H.-Y. Park Choo, S.K. Lee, H.J. Park, C. Kim, S.W. Park, C.-O. Lee, Bioorg. Med. Chem. 12, 3683 (2004)
R. Ghahremanzadeh, G.I. Shakibaei, A. Bazgir, Synlett 8, 1129 (2008)
M.R. Nabid, S.J.T. Rezaei, R. Ghahremanzadeh, A. Bazgir, Ultrason. Sonochem. 17, 159 (2010)
D.S. Raghuvanshi, K.N. Singh, Tetrahedron Lett. 52, 5702 (2011)
H.R. Shaterian, M. Mohammadnia, J. Mol. Liq. 173, 55 (2012)
S.R. Shafe-Mehrabadi, B. Sadeghi, A. Hassanabadi, Polycycl. Aromat. Compd. 42, 574 (2022)
A.G. Mulik, D.R. Chandam, D.R. Patil, P.P. Patil, G.N. Mulik, S.T. Salunkhe, M.B. Deshmukh, Res. Chem. Intermed. 41, 10085 (2015)
A. Chaskar, Current Catalysis. 3, 266 (2014)
N.M. Shah, M.P. Patel, R.G. Patel, J. Heterocycl. Chem. 49, 1310 (2012)
Y.A. Tayade, D.S. Dalal, Catal. Lett. 147, 1411 (2017)
M. Barge, S. Kamble, A. Kumbhar, G. Rashinkar, R. Salunkhe, Monatsh. Chem. 144, 1213 (2013)
A. Patil, M. Barge, G. Rashinkar, R. Salunkhe, Mol. Divers. 19, 435 (2015)
T. Lohar, A. Kumbhar, M. Barge, R. Salunkhe, J. Mol. Liq. 224, 1102 (2016)
G.V. Reddy, S.R. Kanth, D. Maitraie, B. Narsaiah, P.S. Rao, K.H. Kishore, U.S.N. Murthy, B. Ravi, B. Ashok Kumar, T. Parthasarathy, Eur. J. Med. Chem. 44, 1570 (2009)
C.M. Melendez Gomez, V.V. Kouznetsov, M.A. Sortino, S.L. Alvarez, S.A. Zacchino, Bioorg. Med. Chem. 16, 7908 (2008)
G. Li-Ping, J. Qing-Hao, T. Guan-Rong, C. Kyu-Yun, Q. Zhe-Shan, J. Pharma. Pharmaceut. Sci. 10, 254 (2007)
T.C. Ko, M.J. Hour, J.C. Lien, C.M. Teng, K.H. Lee, S.C. Kuo, L.J. Huang, Bioorg. Med. Chem. Lett. 11, 279 (2001)
Acknowledgements
The authors are thankful to the Department of Chemistry, Shivaji University, Kolhapur, and CSIR-National Chemical Laboratory, Pune, for providing spectral data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barge, M., Rashinkar, G., Kanase, D. et al. One-drop organocatalyzed multicomponent synthesis of pyrazolo[1,2-b]phthalazine-diones and pyrazolophthalazinyl quinolines. Res Chem Intermed 48, 5045–5058 (2022). https://doi.org/10.1007/s11164-022-04848-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04848-w