Abstract
Protic ionic liquids (PILs), which are easily produced through the combination of a Brønsted acid and Brønsted base, such as [Mim]Ac and 1,4-diazabicyclo[2.2.2]octane (DABCO):AcOH:H2O (1:1:3), were found to be lucid, tunable tool for synthesis of various heterocyclic motifs such as phthalazinediones, quinoxalines and benzopyrans. These PILs were found to be efficient for synthesis of diverse heterocyclic derivatives, along with demonstrating noteworthy aspects such as high yields, isolation of pure products without column chromatography and recyclable reaction media.
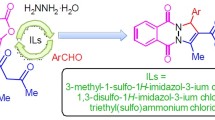
Graphical abstract








Similar content being viewed by others
References
X. Chen, R. Liu, Y. Xu, G. Zou, Tetrahedron 68, 4813 (2012)
E. Janus, I. Goc-Maciejewska, M. Łożyński, J. Pernak, Tetrahedron Lett. 47, 4079 (2006)
J.M. Altimari, J.P. Delaney, L. Servinis, J.S. Squire, M.T. Thornton, S.K. Khosa, B.M. Long, M.D. Johnstone, C.L. Fleming, F.M. Pfeffer, S.M. Hickey, M.P. Wride, T.D. Ashton, B.L. Fox, N.L. Byrne, C. Henderson, Tetrahedron Lett. 53, 2035 (2012)
C.P. Gordon, N. Byrne, A. McCluskey, Green Chem. 12, 1000 (2010)
T.L. Greaves, C.J. Drummond, Chem. Rev. 108, 206 (2008)
T. Welton, Chem. Rev. 99, 2071 (1999)
J.P. Kleim, R. Bender, R. Kirsch, C. Meichsner, A. Paessens, M. Rösne, H.R. Waigmann, R. Kaiser, M. Wichers, K.E. Schneweis, I. Winkler, G. Riess, Antimicrob. Agents Chemother. 39, 2253 (1995)
J. Balzarini, A. Karlsson, C. Meichsner, A. Pessens, G. Riess, E. De Clerq, J.P. Kleim, J. Virol. 68, 1986 (1994)
E.D. Brock, D.M. Lewis, T.I. Yousaf, H.H. Harper, (The Procter and Gamble Company, USA) WO 9951688 (1999)
K.R.J. Thomas, V. Marappan, T.L. Jiann, C. Chang-Hao, T. Yu-ai, Chem. Mater. 17, 1860 (2005)
S. Dailey, J.W. Feast, R.J. Peace, R.C. Saga, S. Till, E.L. Wood, J. Mater. Chem. 11, 2238 (2001)
D. O_Brien, M.S. Weaver, D.G. Lidzey, D.D.C. Bradley, Appl. Phys. Lett. 69, 881 (1996)
L.S. Jonathan, M. Hiromitsu, M. Toshihisa, M.L. Vincent, F. Hiroyuki, Chem. Commun. 8, 862 (2002)
L.S. Jonathan, M. Hiromitsu, M. Toshihisa, M.L. Vincent, F. Hiroyuki, J. Am. Chem. Soc. 124, 13474 (2002)
P.C. Peter, Z. Gang, A.M. Grace, H. Carlos, M.G.T. Linda, Org. Lett. 6, 333 (2004)
O. Sascha, F. Rudiger, Synlett. 15, 1509 (2004)
Kazunobu, T. Ryusuke, O. Tomohiro, M. Shuichi, Chem. Commun. 212 (2002)
S. Louis, M.G. Marc, J.W. Jory, P.B. Joseph, J. Org. Chem. 68, 4179 (2003)
G. Sakata, K. Makino, Y. Kurasawa, Heterocycles 27, 2481 (1988)
S. Grasso, G. DeSarro, N. Micale, M. Zappala, G. Puia, M. Baraldi, C. Demicheli, J. Med. Chem. 43, 2851 (2000)
Y. Nomoto, H. Obase, H. Takai, M. Teranishi, J. Nakamura, K. Kubo, Chem. Pharm. Bull. (Tokyo) 38, 2179 (1990)
N. Watanabe, Y. Kabasawa, Y. Takase, M. Matsukura, K. Miyazaki, H. Ishihara, K. Kodama, H. Adachi, J. Med. Chem. 41, 3367 (1998)
F. Al’-Assar, K.N. Zelenin, E.E. Lesiovskaya, I.P. Bezhan, B.A. Chakchir, Pharm. Chem. J. 36, 598 (2002)
L.F. Tietze, G. Kettschau, Top. Curr. Chem. 189, 12 (1997)
R. Ghahremanzadeh, G.I. Shakibaei, A. Bazgir, Synlett 8, 1129 (2008)
M.R. Nabid, S.J.T. Rezaei, R. Ghahremanzadeh, A. Bazgir, Ultrason. Sonochem. 17, 159 (2010)
H.R. Shaterian, M. Mohammadnia, J. Mol. Liq. 173, 55–61 (2012)
A.G. Mulik, D.R. Chandam, P.P. Patil, D.R. Patil, S.D. Jagdale, M.B. Deshmukh, J. Mol. Liq. 179, 104–109 (2013)
S.V. More, M.N.V. Sastry, C.F. Yao, Green Chem. 8, 91 (2006)
M.M. Heravi, S. Taheri, K. Bakhtiari, H.A. Oskooie, Catal. Commun. 8, 211 (2007)
S.V. More, M.N.V. Sastry, C.C. Wang, C.F. Yao, Tetrahedron Lett. 46, 6345 (2005)
T.K. Huang, R. Wang, L. Shi, X. Lu, Catal. Commun. 9, 1143 (2008)
J.-Y. Liu, J. Liu, J.-D. Wang, D.-Q. Jiao, H.-W. Liu, Synth. Commun. 40, 2047 (2010)
J.-J. Cai, J.-P. Zou, X.-Q. Pan, W. Zhang, Tetrahedron Lett. 49, 7386 (2008)
K. Dhakshinamoorthy, K. Kanagaraj, Pitchumani. Tetrahedron Lett. 52, 69 (2011)
E. Kolvari, M.A. Zolfigol, M. Peiravi, Green Chem. Lett. Rev. 5(2), 155 (2012)
G. Kaupp, M.R. Naimi-Jamal, J. Schmeyers, Tetrahedron 59, 3753 (2003)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
T.S. Jin, A.Q. Wang, X. Wang, J.S. Zhang, T.S. Li, Arkivoc xiv, 78 (2006)
Q. Wu, Y. Xu, H. Zhu, C. Yu, J. Chem. Thermodyn. 49, 87–94 (2012)
Y. Song, H. Ke, N. Wang, L. Wang, G. Zou, Tetrahedron 65, 9086–9090 (2009)
Acknowledgments
We are thankful to the Department of Chemistry, Shivaji University, Kolhapur for providing IR, 1H and 13C NMR Spectral analytical facilities and Balwant College, Vita for providing the laboratory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mulik, A.G., Chandam, D.R., Patil, D.R. et al. Protic ionic liquids: a lucid, rational tool for synthesis of phthalazinediones, quinoxalines and benzopyrans. Res Chem Intermed 41, 10085–10096 (2015). https://doi.org/10.1007/s11164-015-2014-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2014-5