Abstract
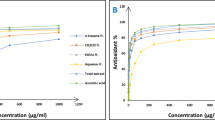
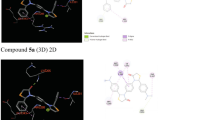
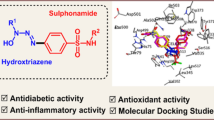
A triazene derivative and its transition metal complexes were prepared and characterized using molar conductance, magnetic susceptibility measurements, IR, UV–visible, NMR spectral studies wherever possible and applicable. The structure of the ligand and metal complexes was further confirmed using DFT calculations with the help of B97d method with 6-311++G(d,p) basis set. The antidiabetic and antioxidant activities of the ligand and the metal complexes were studied. The ligand showed potential biological activities which increased on chelation with metal ion. Apart from this, the molecular docking studies were carried out in order to understand the binding interaction of the ligand and its metal complexes with active sites of the target proteins.

















Similar content being viewed by others
References
D.B. Kimball, M.M. Haley, Angew. Chem. Int. Ed. 41, 3338 (2002)
B.R. Henke, T.G. Consler, N. Go, J. Med. Chem. 45, 5492 (2002)
V.K. Pandey, S. Tusi, Z. Tusi, M. Joshi, S. Bajpai, Acta Pharm. 54, 1 (2004)
D.F. Back, M. Horner, F. Broch, G.M. de Oliveira, Polyhedron 31, 558 (2012)
L. D. Quin, J. Tyrell, Fundamentals of heterocyclic chemistry: Importance in nature and in the synthesis of pharmaceuticals, Wiley-Interscience, 2010
C.O. Kappe, Tetrahedron 49, 6937 (1993)
G. Meng, Y. Liu, A. Zheng, F. Chen, W. Chen, Eur. J. Med. Chem. 82, 600 (2014)
S. Wild, G. Roglic, A. Green, R. Sicree, H. King, Diabetes Care 27, 1047 (2004)
R.M. Poole, R.T. Dungo, Drugs 74, 611 (2014)
K. Gewald, E. Chinke, H. Bottcher, Chem. Ber. 99, 94 (1966)
E. Apostolidis, Y.I. Kwon, K. Shetty, Innov. Food. Sci. Emerg. Technol. 8, 46 (2007)
M. Oyaizu, J. Nutr. 44, 307 (1986)
D.C Garrat, The Quantitative analysis of Drugs. Chapman and Hall Ltd., Japan, 3, 456 (1964)
W.J. Geary, Coord. Chem. Rev. 7, 81 (1971)
J. Wang, H. Niino, A. Yabe, Appl. Surf. Sci. 154, 571 (2000)
S.I. Mostafa, M.A. Kabil, E.M. Saad, A.A. El-Asmy, J. Coord. Chem. 59, 279 (2006)
M. Thankamony, S.B. Kumari, G. Rijulal, K. Mohanan, J. Therm. Anal. Cal. 95, 259 (2009)
K.V. Sharma, V. Sharma, R.K. Dubey, U.N. Tripathi, J. Coord. Chem. 62, 493 (2009)
R. M. Shaker, M. A Elrady, K.U Sadek, Mol. Divers. 2016, 20,153 (2016)
H.M. Wen, Y.H. Wu, Y. Fan, L.Y. Zhang, C.N. Chen, Z.N. Chen, Inorg. Chem. 249, 2210 (2010)
A. Arab, M. Habibzadeh, Comput. Theor. Chem. 1068, 52 (2015)
R.G. Pearson, Proc. Natl. Acad. Sci. 83, 8440 (1986)
P. Politzer, J.S. Murray, Theor. Chem. Acc. 108, 134 (2002)
S. Murray, P. Politzer, Comput. Mol. Sci. 2, 153 (2011)
S.Q. Zhang, F.L. Jiang, M.Y. Wu, L. Chen, J.H. Luo, M.C. Hong, Cryst. Eng. Comm. 15, 3992 (2013)
W. Zhang, W. Dou, W.S. Liu, X.L. Tang, W.W. Qin, Eur. J. Inorg. Chem. 5, 748 (2011)
A.L. Dawidowicz, D. Wianowska, M. Olszowy, Food Chem. 131, 1037 (2012)
R.E. Huie, S. Padmaja, Free Radical Res. Commun. 18, 1951 (1993)
M.N. Patel, D.S. Gandhi, P.A. Parmar, Inorg. Chem. Commun. 13, 618 (2010)
H.Q. Qurrat-ul-Ain, A. Nadhman, M. Sirajuddin, Inorg. Chim. Acta 423, 220 (2014)
M. Taha, M.S. Baharudin, N.H. Ismail, S. Imran, M.N. Khan, F. Rahim, M. Selvaraj, S. Chigurupati, M. Nawaz, F. Qureshii, S. Vijayabalan, Bioorg. Chem. 80, 36 (2018)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Philip, S., Jayasree, E.G. & Mohanan, K. Antidiabetic, antioxidant, DFT and molecular docking studies of a triazene derivative and its transition metal complexes. Res Chem Intermed 46, 75–99 (2020). https://doi.org/10.1007/s11164-019-03936-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03936-8