Abstract
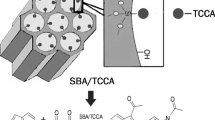
SBA/hydrotalcite/heteropolyacid nanocomposite is synthesized via a novel procedure in which the as-prepared heteropolyacid-loaded SBA-15 was impregnated with calcined hydrotalcite. The ternary hybrid system was characterized by using SEM/EDS, XRD, BET, TPD, TGA, FTIR and ICP-AES and also used as an efficient catalyst for the synthesis of 2,4-dihydro-3H-pyrazol-3-one derivatives via the reaction of arylaldehydes and 5-methyl-1H-pyrazol-3(2H)-one under reflux condition. Moreover, the catalytic activity of this catalyst was confirmed for the one-pot four-component reaction of aryl aldehydes, ethylacetoacetate, malononitrile and hydrazine hydrate/phenyl hydrazine in aqueous media for the synthesis of pyranopyroles. The investigation of the effect of the synthetic procedure and calcination of the hydrotalcite on the catalytic activity of the catalyst established that this factor does not exert a marked effect on the catalytic activity. The present procedures benefit from diverse advantages, including high yields, simplicity, mild reaction conditions and short reaction times. Moreover, this catalyst was reusable for up to five reaction runs and the HPA leaching was suppressed.














Similar content being viewed by others
References
S. Rostamnia, E. Doustkhah, RSC Adv. 4, 28238 (2014)
B. Karimi, F. Mansouri, M. Khorasani, Curr. Org. Chem. 20, 349 (2016)
L. Konga, X. Zhoua, Y. Yaoa, P. Jiana, G. Diaoa, Environ. Technol. 37, 422 (2016)
H. Veisi, A.A. Manesh, N. Eivazi, A.R. Faraji, RSC Adv. 5, 20098 (2015)
H. Veisia, M. Hameliana, S. Hemmati, J. Mol. Catal. A Chem. 395, 25 (2014)
A. Wach, M. Drozdek, B. Dudek, P. Latka, P. Kustrowski, Microporous Mesoporous Mater. 226, 433 (2016)
X. Song, W. Zhu, K. Li, J. Wang, H. Niu, H. Gao, W. Gao, W. Zhang, J. Yu, M. Jia, Catal. Today 259, 59 (2016)
S. Sadjadi, M.M. Heravi, RSC. Adv. 7, 30815 (2017)
J. Zhang, P. Jiang, Y. Shen, W. Zhang, G. Bian, J. Porous Mater. 23, 431 (2016)
V. Mahdavi, M. Mardani, Res. Chem. Intermed. 41, 8907 (2015)
R.L. Oliveira, W. He, R.J.M. Klein Gebbink, K.P. de Jong, Catal. Sci. Technol. 5, 1919 (2015)
D. Saikia, Y.-Y. Huang, C.-E. Wu, H.-M. Kao, RSC Adv. 6, 35167 (2016)
X. Sheng, Y. Zhou, Y. Yang, Y. Zhang, Z. Zhang, S. Zhou, X. Fu, S. Zhao, RSC Adv. 4, 30697 (2014)
S. Sadjadi, M.M. Heravi, Curr. Org. Chem. 20, 1404 (2016)
J.J. Walsh, A.M. Bond, R.J. Forster, T.E. Keyes, Coord. Chem. Rev. 306, 217 (2016)
L. Hong, Y. Gui, J. Lu, J. Hu, J. Yuan, L. Niu, Int. J. Hydrog. Energy 38, 11074 (2013)
Y. Zhu, M. Zhu, L. Kang, F. Yu, B. Dai, Ind. Eng. Chem. Res. 54, 2040 (2015)
J. Qu, Q. Zhang, X. Li, X. He, S. Song, Appl. Clay 119, 185 (2016)
S. Omwoma, W. Chen, R. Tsunashima, Y.-F. Song, Coord. Chem. Rev. 258–259, 58 (2014)
S.N. Basahel, S.A. Al-Thabaiti, K. Narasimharao, N.S. Ahmed, M. Mokhtar, J. Nanosci. Nanotechnol. 14, 1931 (2014)
W. Guo, Y. Zhao, F. Zhou, X. Yan, B. Fan, R. Li, Appl. Catal. A 522, 101 (2016)
T. Baskaran, J. Christopher, A. Sakthivel, RSC Adv. 5, 98853 (2015)
D. Pan, H. Zhang, T. Fan, J. Chen, X. Duan, Chem. Commun. 47, 908 (2011)
C.O. Kappe, Acc. Chem. Res. 33, 879 (2000)
M.D. Burke, S.L. Schreiber, Angew. Chem. Int. Ed. 43, 46 (2004)
J. Lu, P.H. Toy, Chem. Rev. 109, 815 (2009)
A. Rahmati, Z. Khalesi, Tetrahedron 68, 8472 (2012)
S.V.N. Vuppalapati, Y.R. Lee, Tetrahedron 68, 8286 (2012)
C.-J. Li, T.-H. Chan, Comprehensive organic reactions in aqueous media (Wiley, New York, 2007)
A. Chanda, V.V. Fokin, Chem. Rev. 109, 725 (2009)
A. Schmidt, A. Dreger, Curr. Org. Chem. 15, 1423 (2011)
A.N. Kost, I.I. Grandberg, Progress in pyrazole chemistry, in Advances in Heterocyclic Chemistry, vol. 6, ed. by A.R. Katritzky, A.J. Boulton (Academic Press, Cambridge, 1966), p. 347
W.S. Hamama, Synth. Commun. 31, 1335 (2001)
W. Wang, S.X. Wang, X.Y. Qin, J.T. Li, Synth. Commun. 35, 1263 (2005)
D. Singh, D. Singh, J. Chem. Eng. Data 29, 355 (1984)
K. Niknam, D. Saberi, M. Sadegheyan, A. Deris, Tetrahedron Lett. 51, 692 (2010)
D.Q. Shi, J. Chen, N. Wu, Q.Y. Zhuang, X.S. Wang, Chin. J. Org. Chem. 25, 405 (2005)
K. Sujatha, G. Shanthi, N.P. Selvam, S. Manoharan, P.T. Perumal, M. Rajendran, Bioorg. Med. Chem. Lett. 19, 4501 (2009)
M.N. Nasr, M.M. Gineinah, Arch. Pharm. 335, 289 (2002)
M.A. Zolfigol, M. Tavasoli, A.R. Moosavi-Zare, P. Moosavi, H.G. Kruger, M. Shiri, V. Khakyzadeh, RSC Adv. 3, 25681 (2013)
V.K. Ahluwalia, A. Dahiya, V.K. Garg, Indian J. Chem. Sect. B. 36, 88 (1997)
N.R. Mohamed, N.Y. Khaireldin, A.F. Fahmy, A.A. El-Sayed, Der Pharma Chem. 2, 400 (2010)
H. Junek, H. Aigner, Chem. Ber. 106, 914 (1973)
Y.A. Sharanin, L.G. Sharanina, V.V. Puzanova, Chem. Informationsdienst, 19, 2609–2615 (1983)
G. Vasuki, K. Kumaravel, Tetrahedron Lett. 49, 5636 (2008)
M.M. Heravi, E. Hashemi, Y. Shirazi Beheshtiha, Sh Ahmadi, T. Hosseinnejad, J. Mol. Catal. A. 394, 74 (2014)
M.M. Heravi, F. Mousavizadeh, N. Ghobadi, M. Tajbakhsh, Tetrahedron Lett. 55, 1226 (2014)
S.S. Sadjadi, M.M. Heravi, V. Zadsirjan, V. Farzaneh, Appl. Surf. Sci. 426, 889 (2017)
M. Hami Dindar, M.R. Yaftian, M. Hajihasani, S. Rostamnia, J. Taiwan Inst. Chem. Eng. 67, 325 (2016)
A. Aouissi, Z.A. Al-Othman, H. Al-Anezi, Molecules 15, 3319 (2010)
T. Baskaran, J. Christopher, T.G. Ajithkumar, A. Sakthivel, Appl. Catal. A. 488, 119 (2014)
V. Fathi Vavsari, G. Mohammadi Ziarani, S. Balalaie, A. Latifi, M. Karimi, A. Badiei, Tetrahedron 72, 5420 (2016)
S.K. Sharma, P.K. Kushwaha, V.K. Srivastava, S.D. Bhatt, R.V. Jasra, Ind. Eng. Chem. Res. 46, 4856 (2007)
B. Wiyantoko, P. Kurniawati, T.E. Purbaningtias, I. Fatimah, Procedia Chem. 17, 21 (2015)
Z. Wang, F. Liu, C. Lu, Chem. Commun. 47, 5479 (2011)
S. Mallik, S.S. Dash, K.M. Parida, B.K. Mohapatra, J. Colloid Interface Sci. 300, 237 (2006)
K.D. Parghi, J.R. Satam, R.V. Jayaram, Green Chem. Lett. Rev. 4, 143 (2011)
K. Fan, J. Liu, X. Yang, L. Rong, RSC Adv. 5, 37916 (2015)
Y. Peng, G. Song, R. Dou, Green Chem. 8, 573 (2006)
M. Mirza-Aghayan, F. Saravani, A.A. Tarlani, M.S. Abaee, R. Boukherroub, Monatsh. Chem. 144, 1699 (2013)
H. Sheibani, M. Babaie, Synth. Commun. 40, 257 (2009)
J. Deng, L.-P. Mo, F.-Y. Zhao, Z.-H. Zhang, S.-X. Liu, ACS Comb. Sci. 14, 335 (2012)
K. Kanagaraj, K. Pitchumani, Tetrahedron Lett. 51, 3312 (2010)
A. Siddekha, A. Nizam, M.A. Pasha, Spectrochim. Acta Part A 81, 431 (2011)
M.A.E.A.A. Ali, E. Remaily, S.K. Mohamed, Tetrahedron 70, 270 (2014)
K. Ablajan, W. Liju, A. Tuoheti, Y. Kelimu, Lett. Org. Chem. 9, 639 (2012)
M. Babaie, H. Sheibani, Arab. J. Chem. 4, 159 (2011)
H. Mecadon, M.R. Rohman, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 2523 (2011)
R.-Y. Guo, Z.-M. An, L.-P. Mo, S.-T. Yang, H.-X. Liu, S.-X. Wang, Z.-H. Zhang, Tetrahedron 69, 9931 (2013)
A. Vafaee, A. Davoodnia, M. Pordel, Res. Chem. Intermed. 41, 8343 (2015)
Acknowledgements
The authors appreciate partial financial supports from Alzahra University and Iran Polymer and Petrochemical Institute. MMH is also thankful to the Iran National Science Foundation for the individual given grant.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sadjadi, S., Heravi, M.M., Zadsirjan, V. et al. A ternary hybrid system based on combination of mesoporous silica, heteropolyacid and double-layered clay: an efficient catalyst for the synthesis of 2,4-dihydro-3H-pyrazol-3-ones and pyranopyrazoles in aqueous medium: studying the effect of the synthetic procedure on the catalytic activity. Res Chem Intermed 44, 6765–6785 (2018). https://doi.org/10.1007/s11164-018-3521-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3521-y