Abstract
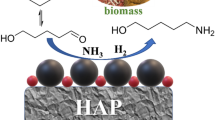
Poly(N-vinyl-2-pyrrolidone)-capped ruthenium-supported hydroxyapatite (Ru-PVP/HAP) shows significant activity for the synthesis of furfurylamine (FAM) via the reductive amination of furfural. As-prepared 5 wt% Ru-PVP/HAP affords 50 % yield of FAM in 25 % NH3 aqueous solution under pressurized H2 gas (2.5 atm), and the highest yield approaches 60 % at 4.0 H2 atm. Comparison between the activities over four Ru-supported HAP catalysts prepared with different methods and the results of X-ray absorption spectroscopy suggested that the metallic Ru cluster is the active center for the reductive amination of furfural. Transmission electron microscope and inductively-coupled plasma analysis indicated that the as-prepared 5 wt% Ru-PVP/HAP catalyst possessed 4.0 wt% PVP-capped Ru clusters with average diameter of 1.7 ± 0.3 nm on HAP support. It was also demonstrated that the reductive amination approach with Ru-PVP/HAP catalyst, NH3 aq. and pressurized H2 gas has capability for transformation of aromatic aldehydes to the corresponding aromatic amines. According to these results, it is concluded that Ru(0) cluster supported on HAP will represent a suitable catalyst for widely-usable reductive amination to convert an aldehyde functionality towards an amine.









Similar content being viewed by others
Notes
As intermediate, hydrofuramide (Exact Mass: 268.08) is the principal candidate estimated by GC–MS(EI) analysis (see Fig. S7).
The peaks at 27.3 and 28.5 min were not originated from tetrahydro-FAM (Exact mass: 101.08) nor di-furfurylamine (Exact mass: 177.08) estimated by GC–MS(EI) analysis. Thus, side-reactions in our condition have not been identified at this moment.
References
J.P. Lange, E. der Heide, J. van Buijtenen, R. Price, ChemSusChem 5, 150 (2012)
S. Dutta, S. De, B. Saha, M.I. Alam, Catal. Sci. Technol. 2, 2025 (2012)
M. Vilches-Herrera, S. Werkmeister, K. Junge, A. Borner, M. Beller, Catal. Sci. Technol. 4, 629 (2014)
T. Mitsudome, Y. Mikami, M. Matoba, T. Mizugaki, K. Jitsukawa, K. Kaneda, Amgew. Chem. Int. Ed. 51, 136 (2015)
E.H. Boymans, P.T. Witte, D. Vogt, Catal. Sci. Technol. 5, 176 (2015)
C.F. Wang, J.Q. Sun, X.D. Liu, A. Sudo, T. Endo, Green Chem. 14, 2799 (2012)
L.B. Maktouf, I. Ghorbel, A. Afli, S. Abid, A. Gandini, Polym. Bull. 67, 1111 (2011)
M.S. Holfinger, A.H. Conner, D.R. Holm, C.G. Hill Jr, J. Org. Chem. 60, 1595 (1995)
M.A. Ayedi, Y.L. Bigot, H. Ammar, S. Abid, R.E. Gharbi, M. Delmas, Synth. Commun. 43, 2127 (2013)
M.A. Ayedi, Y.L. Bigot, H. Ammar, S. Abid, R.E. Gharbi, M. Delmas, J. Soc. Chim. Tuniste 14, 109 (2012)
T. Ayusawa, S. Mori, T. Aoki, R. Hamana, US Patent No. US4598159A (1984)
Z. Binggeng, China Patent No. CN1704411A (2005)
S. Nishimura, Y. Yakita, M. Katayama, K. Higashimine, K. Ebitani, Catal. Sci. Technol. 3, 351 (2013)
M. Mohammad, S. Nishimura, K. Ebitani, AIP Conf. Proc. 1649, 58 (2015)
K. Yamaguchi, T. Koike, J.W. Kim, Y. Ogasawara, N. Mizuno, Chem. Eur. J. 14, 11480 (2008)
M. Zawadzki, J. Okal, Mater. Res. Bull. 43, 3111 (2008)
J. Shen, X. Yin, D. Karpuzov, N. Semagina, Catal. Sci. Technol. 3, 208 (2013)
M. Sadakiyo, M. Kon-no, K. Sato, K. Nagaoka, H. Kasai, K. Kato, M. Yamauchi, Dalton Trans. 43, 11295 (2014)
C. Sly, US Patent No. 2112715A (1938)
G.D. Varlamov, Y.M. Mamatov, A.S. Shashkov, Chem. Heterocycl. Comp. 14, 1184 (1978)
R. Schiff, Ber. Dtsch. Chem. Ges. 10, 1186 (1877)
C.F. Winans, J. Am. Chem. Soc. 61, 3566 (1939)
Y. Hachihama, M. Imoto, H. Sumitomo, E. Negoro, KOGYOKAGAKUZASSHI 53, 24 (1950)
M. Tamura, K. Tomishige, Angew. Chem. Int. Ed. 54, 864 (2015)
L.L. Chng, J. Yang, J.Y. Ying, ChemSusChem 8, 1916 (2015)
Y. Li, I. Sorribes, T. Yan, K. Junge, M. Beller, Angew. Chem. Int. Ed. 52, 12156 (2013)
K. Shimizu, Y. Miyamoto, T. Kawasaki, T. Tanji, Y. Tai, A. Satsuma, J. Phys. Chem. C 113, 17803 (2009)
J. Tuteja, S. Nishimura, K. Ebitani, RSC Adv. 4, 38241 (2014)
T. Gross, A.M. Seayad, M. Ahmad, M. Beller, Org. Lett. 4, 2055 (2002)
R.-Y. Lai, C.-I. Lee, S.-T. Liu, Tetrahedron 64, 1213 (2008)
A.W. Heinene, J.A. Peters, H. van Bekkum, Eur. J. Org. Chem. 2000, 2501 (2000)
S. Gomez, J.A. Peters, J.C. van der Waal, T. Maschmeyer, Appl. Catal. A:Gen. 254, 77 (2003)
Acknowledgments
This work is supported by the Grant-in-Aid for Scientific Research (C) (No. 25420825) and Young Scientist (B) (No. 25820392) by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The authors also appreciate the contribution of Photon Factory at High Energy Accelerator Research Organization (KEK-PF), Tsukuba, Japan, on XAFS discussions at BL-9A under the Proposal No. 2013G586.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nishimura, S., Mizuhori, K. & Ebitani, K. Reductive amination of furfural toward furfurylamine with aqueous ammonia under hydrogen over Ru-supported catalyst. Res Chem Intermed 42, 19–30 (2016). https://doi.org/10.1007/s11164-015-2334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2334-5