Abstract
Climate change has increased the susceptibility of forest ecosystems, resulting in escalated forest decline globally. As one of the largest forest biomasses in the Northern Hemisphere, the Eurasian boreal forests are subjected to frequent drought, windthrow, and high-temperature disturbances. Over the last century, bark beetle outbreaks have emerged as a major biotic threat to these forests, resulting in extensive tree mortality. Despite implementing various management strategies to mitigate the bark beetle populations and reduce tree mortality, none have been effective. Moreover, altered disturbance regimes due to changing climate have facilitated the success of bark beetle attacks with shorter and multivoltine life cycles, consequently inciting more frequent bark beetle-caused tree mortality. This review explores bark beetle population dynamics in the context of climate change, forest stand dynamics, and various forest management strategies. Additionally, it examines recent advancements like remote sensing and canine detection of infested trees and focuses on cutting-edge molecular approaches including RNAi-nanoparticle complexes, RNAi-symbiotic microbes, sterile insect technique, and CRISPR/Cas9-based methods. These diverse novel strategies have the potential to effectively address the challenges associated with managing bark beetles and improving forest health in response to the changing climate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the last several decades, a shift in climatic conditions has increased the frequency of severe windstorms and drought events, favouring the emergence and proliferation of insect outbreaks. Bark beetles (Coleoptera: Curculionidae: Scolytinae) are widespread globally, and several species are associated with extensive mortality of major tree species in the Northern Hemisphere (Vega and Hofstetter 2015; Hlásny et al. 2021). For instance, the Eurasian spruce bark beetle, Ips typographus (L.) plays a vital role in forest succession and nutrient recycling by decomposing dead and dying trees (Edmonds and Eglitis 1989; Hofstetter et al. 2015; Raffa et al. 2015). However, once their population density reaches the epidemic threshold, they have the potential to cause widespread forest mortality (Berryman et al. 1989; Hlásny et al. 2021). These outbreaks have gone beyond economic implications by disrupting forest succession and nutrient cycling and turning forests from carbon sink to source (Seidl et al. 2014; Aldea et al. 2023).
As the primary host for I. typographus and one of the major tree species in the Eurasian boreal forests, Norway spruce (Picea abies) is highly vulnerable to rising temperatures. For instance, unprecedented Norway spruce mortality occurred from 1970 to 2010 due to frequent drought events and heatwaves, which resulted in an average annual loss of up to 14 million m3 per annum, especially 118 million m3 in 2019 alone (Ebner 2020). Furthermore, its primary pest, I. typographus has benefited from warming temperatures, which resulted in accelerated and extended brood development and early swarming (Hlásny et al. 2011; Netherer et al. 2014; Hinze and John 2020).
Management of bark beetles is driven by two primary objectives: prevention and containment (Wermelinger 2004). Prevention strategies aim to keep the beetle populations at low densities by implementing measures such as removing infested trees, employing traps, and clearing fallen trees. Containment involves salvage and sanitation logging in outbreak areas or nearby areas to halt or slow the spread of outbreaks. However, the current management strategies are still inadequate, and their effectiveness has been frequently questioned (Dobor et al. 2020). Consequently, exploring innovative strategies for bark beetle management is imperative, leveraging techniques emerging from functional genomics. Methods such as RNA interference (RNAi) and CRISPR/Cas9 are rapidly advancing as pest control tools, with their effectiveness proven in controlling agricultural pests, such as Spodoptera and the Colorado potato beetle (Gui et al. 2020; Mezzetti et al. 2020; Vatanparast and Park 2022). Recent reports demonstrate the presence of core machinery genes that can be utilized for gene silencing for Ips management using molecular tools (Powell et al. 2021; Joga et al. 2021). However, previous reports have not highlighted or addressed the applicability of these techniques. This review emphasizes the future usability and efficiency of such techniques in Ips bark beetle management. Unlike many conventional pest management approaches, these molecular methods are tailored to target specific species. Nevertheless, they require further exploration and functional validation for I. typographus management.
Despite a century of extensive research on bark beetles, there are several gaps in our understanding of the main drivers of bark beetle outbreak dynamics and the strategies for their management. Moreover, the recent advancements in the bark beetle management techniques are poorly elaborated. Here, we aim to provide a brief overview of the ecology and population dynamics of I. typographus and discuss the major drivers of outbreaks in the context of climate change. This overview focuses on I. typographus, but we also discuss other eruptive bark beetle species. We preliminarily examine how changes in a forest structure affect the initial host colonization and spread, how droughts and water stress affect host tree physiology and vigour, and how temperature regimes affect bark beetle activity. We analysed the present management approaches and their efficacy in protecting the forests and suggested cutting-edge molecular-based approaches to face new challenges that could be used to guide future forest management practices in the Anthropocene.
2 Eurasian spruce bark beetle ecology
Ips typographus is a relatively small insect (4.5–5 mm) and exhibits sexual dimorphism. They are univoltine (producing one generation per year) but may shift to polyvoltine (more than two generations per year) due to the influence of warm temperatures, resulting in accelerated development (Hlásny et al. 2011). During the endemic phase, the beetles infest mature trees that are stressed and weakened due to various biotic or abiotic factors (e.g., high temperature, drought, disease, herbivory). The beetles are associated with a variety of microbial symbionts, including bacteria and ophiostomoid fungi, that play a significant role in exhausting tree defenses (Lieutier et al. 2009; Zhao et al. 2019b; Chakraborty et al. 2023). After overwintering, a new generation of beetles emerge from the litter or Norway spruce trunks and disperses between 100 m to tens of kilometers while searching for a suitable host tree (Biedermann et al. 2019; Fig. 1A). Host tree-bark beetle interaction is generally considered multifaceted, and various factors across different spatial and temporal scales shape their interactions (Jakuš et al. 2011; Kautz et al. 2011; Netherer et al. 2024). Furthermore, Ips typographus shows density-dependent host colonization behaviour. Beetle populations in the endemic phase rely on the availability of windthrown, dead, or weakened trees; in contrast, in the epidemic phase, they colonize healthy trees (Økland and Berryman 2004; Økland and Bjørnstad 2006). We will review factors in the context of the population dynamics of I. typographus.

(Adapted from Kautz et al. 2014). (B.) a. The initial host colonization stage is usually initiated by pioneer beetles, males in Ips and females in Dendroctonus. In Ips typographus, aggregation pheromones, 2-methyl-3-buten-2-ol (MB) and (-)-cis-verbenol (cV) are released to attract additional male and female beetles. b. If the pioneering beetle survives host tree defenses, with the arrival of additional beetles, they produce larger amounts of aggregation pheromones which usually ends the mass colonization of host trees. The goal of mass colonization is to exhaust tree defense to secure reproduction. c. During the mass colonization stage, while the initial tree (I) is mass colonized, a small portion of male beetles land on a neighbouring uncolonized tree (II) and starts a new colonization sequence. d. As the production of attractive pheromone components diminishes, the attraction towards the initial tree declines, while simultaneously, attraction towards the neighbouring tree increases, starting a switch in attraction. e. When the initial "switching" process is completed, only small amounts of aggregation pheromones are produced, while larger amounts of inhibitory compounds (ipsdienol & ipsenol) are continuously produced. The attraction is now shifted to the neighbouring tree (II), where a new mass attack starts, with a small proportion of males once more being deflected and ending up on the new uncolonized neighbouring tree (III). The small graphs represent log pheromone release from Birgersson et al. (1984). The marks on the x-axis represent the attack phases 1, 3 and 6, where (1) males are bored in bark, (3) have completed nuptial chamber formation, and (6) are joined with females. (Adapted from Schlyter et al. 1987)
Scheme of Ips typographus population dynamics. (A.) In the endemic phase, beetles feed and reproduce in windthrown trees, trunks, and stumps and later move to the epidemic phase under favourable conditions. There are several factors, such as favourable weather, availability of breeding substrate, droughts, and windthrows, that influence the transition of the beetle populations to the epidemic phase. In the epidemic phase, the beetle population is high enough to mass attack healthy trees and cause widespread mortality. Later, certain factors such as unfavourable weather, exhaustion of the breeding substrate, abundance of predators, and increased tree resistance of surviving trees can cause high beetle mortality and limit the population to the endemic phase
2.1 Stand-level dynamics
At the stand level, once the pioneer male beetles overcome the host defenses, they produce aggregation pheromones, 2-methyl-3-buten-2-ol (MB) and (-)-cis-verbenol (cV) (Keeling et al. 2021; Ramakrishnan et al. 2022; Fig. 1B.a). Male beetles produce high amounts of MB when initiating the nuptial chamber, while the production of cV changes depending on the amount and enantiomeric composition of α-pinene in Norway spruce phloem (Birgersson et al. 1984; Fig. 1B.b). Emission of these aggregation pheromones from trees under attack brings additional male and female beetles to the foci trees. Arriving male and female conspecifics release more of the same pheromones to coordinate mass attacks on the host (Blomquist et al. 2010). Upon entering the host, adult beetles create mating chambers, mate, and excavate oviposition galleries where eggs are deposited. Eggs hatch into larvae, feed, complete their development under bark, and exit from the parental trees. Depending on the host characteristics, such as phloem thickness, I. typographus can increase its population size by up to 15-fold from the preceding generation (Hlásny et al. 2019).
Once the foci host trees are fully occupied, the production of anti-attractant compounds such as verbenone starts to avoid overcrowding (Xu et al. 2015) while the production of aggregation pheromones declines. This increases attraction towards nearby uninfested trees, initiating a new wave of mass attacks (Fig. 1B.c, 1B.d). This event marks the completion of “switching” of bark beetle attacks from heavily colonized trees to neighbouring uncolonized trees (Schlyter et al. 1987; Fig. 1B.e). This process may result in several mass-attacked trees at the landscape level.
2.2 Landscape-level dynamics
The spatial distribution of mass-attacked trees, the host availability, and environmental factors can influence the initiation and spread of bark beetle infestations. However, the effects of these factors vary between endemic and epidemic phases of I. typographus (Mezei et al. 2014a; Potterf et al. 2019).
2.2.1 Endemic phase
When bark beetle population densities are low, they are primarily associated with stressed or defensively compromised trees (Raffa et al. 2008; Boone et al. 2011; Biedermann et al. 2019). Factors such as tree resistance, stand structure, abundance of natural enemies and competitors, and weather can potentially limit the beetle’s population size at the endemic phase, whereas natural disturbances and climatic events can disrupt this equilibrium by reducing tree resistance and hence increasing the beetle population (Raffa et al. 2008; Ruel et al. 2023). The capacity of bark beetle populations to cross the epidemic threshold and move from the endemic to the outbreak phase depends critically on the availability of suitable breeding materials, such as susceptible host trees, stumps, and windblown trees (Hroššo et al. 2020). Weather events like extensive wind damage or intense droughts can provide ample breeding grounds for population build-up. As the population has built up and depleted the available substrate, they begin to attack healthier trees (Biedermann et al. 2019).
2.2.2 Epidemic (outbreak) phase
Once the beetle population reaches the epidemic phase, the discriminatory behaviour in host selection becomes less adaptive because there is a greater possibility of recruiting sufficient numbers of conspecifics to overcome the defenses of large healthy trees, which tend to have greater resources (i.e., thicker phloem) to support substantial beetle populations, resulting in higher number of broods (Raffa et al. 2008). The factors governing the bark beetle outbreaks also change as the infestation progresses (Walter and Platt 2013).
In the early stages of the outbreak, beetles still infest susceptible trees with weakened defenses (Raffa et al. 2008), with most beetles targeting windthrown trees. As a result, the beetle infestation is still relatively small at this stage, and tree mortality is primarily attributed to windthrow events (Potterf et al. 2019). However, the infestation of standing trees intensifies up to three years after a major windthrow event and enters a patch-driven outbreak phase, signified by the establishment of many infestation spots, benefiting from a large pool of available healthy host trees (Økland et al. 2016; Potterf et al. 2019). Still, the rate of infestation spread is minimal, and the distance between new and old infestation sites is maximum (Jakuš et al. 2002). These spots typically emerge close to windthrow areas within previously unaffected stands, appearing as small, rounded patches (Potterf et al. 2019).
As the epidemic spreads across the landscape, beetle pressure becomes the most crucial determinant of tree mortality because the population size of bark beetles is large enough to mass attack healthy trees and overcome their defenses (Walter and Platt 2013; Mezei et al. 2014b). The outbreak emerges within one to three years following a major disturbance event (Økland and Berryman 2004). Although healthy trees can withstand a certain number of attacks, once a certain threshold is surpassed, such a tree cannot repel the attackers (Krokene 2015). As a result of the coalescence of nearby infestation spots, the distance between freshly and previously infested locations is smaller during the epidemic phase than the endemic phase (Kautz et al. 2011; Potterf et al. 2019). As each successive generation of bark beetles exhausts resources needed by subsequent generations (Raffa et al. 2008), higher population pressure further facilitates the initiation and spread of bark beetle infestations at higher elevations (Jakuš et al. 2002) and other neighbouring less affected areas.
2.2.3 Epidemic to the endemic phase transition
In the later phase of the outbreak, as the significant portion of available host trees has already been depleted, bark beetles target marginal host trees in less favourable habitats. This phase is characterized by relatively lower densities of beetle populations than the earlier phases of the outbreak, and the beetle numbers are too low to kill healthy trees. Surviving trees are likely to fend off attacking beetles due to their anatomical and chemical defenses (Nelson et al. 2007; Raffa et al. 2008; Erbilgin et al. 2017; Zhao and Erbilgin 2019; Zhao et al. 2019a). In the post-epidemic phase, while the number of new bark beetle infestation sites decreases, the expansion of existing infestation spots reaches its maximum, with the beetles more likely to target less suitable resources neighbouring old spots due to limited available resources.
The mechanisms behind the decline in bark beetle populations in the later stage of an outbreak are not well understood. However, when the host tree supply is depleted, the beetle population decreases significantly (Walter and Platt 2013). Additionally, adverse weather conditions, pathogens, and natural enemies can significantly increase beetle mortality, ultimately limiting their population to the endemic phase (Boone et al. 2011; Wegensteiner et al. 2015).
3 Effects of forest stand structure on population dynamics of I. typographus
The structure of the forest stand (e.g., stand density and age classes) strongly influences bark beetle outbreak (Fettig et al. 2007; Sproull et al. 2015). Windthrow alters stand structure as a primary form of disturbance by creating openings in the forest canopy (Marini et al. 2017). Promoting forest diversity (discussed in Sect. 6.2) and thinning are the highly recommended practices to reduce overall vulnerability and have been effective against Dendroctonus ponderosae, D. frontalis, and D. brevicomis (Egan et al. 2010; Fettig and McKelvey 2010; Zhang et al. 2013; Hood et al. 2016).
Within outbreak stands, foresters employ intensive measures like sanitation felling and salvage logging to reduce damage, including cutting and removing infested or dead trees (Stadelmann et al. 2013). However, intensive management activities such as sanitation can further amplify the destabilization of the stand. For example, intense sanitary interventions in outbreak-impacted stands could cause the fragmentation of forest stands by creating canopy openings and numerous margins or edges (Hanson and Lorimer 2007; Zabihi et al. 2021; Özçelik et al. 2022; Singh et al. 2023). Notably, fragmented forests exhibit increased susceptibility to wind damage and render freshly formed forest edges to bark beetle infestation (Kautz et al. 2013; Marešová et al. 2020). This susceptibility is likely due to sudden changes in microclimatic conditions, such as increased solar radiation and temperature, reduced humidity, increased vapour pressure demands, and greater wind exposure (Hanson and Lorimer 2007; Herbst et al. 2007; Marešová et al. 2020; Özçelik et al. 2022). The resulting changes may further facilitate increased bark beetle generations and temporal scale of infestation and increase the damage risk.
Likewise, highly dense plantations, often driven by commercial goals, exhibit poor growth and resilience to stress. Increased tree density can increase intraspecific competition among individual trees and constrain resource availability (Zabihi et al. 2023; Thomas et al. 2024). Research indicates that increased competition within a forest stand can exacerbate the impacts of drought and increase the likelihood of mortality, particularly if water is the limiting resource (Zhang et al. 2015; Young et al. 2017; Korolyova et al. 2022). Consequently, constraints on resource availability can influence many plant functions, such as resin exudation, photosynthesis, and subsequent biosynthesis of carbon-dependent defense metabolites (Bazzaz et al. 1987; Netherer et al. 2014; Erbilgin et al. 2021), and predispose them to bark beetle attacks (Netherer et al. 2019).
Practices like thinning can enhance forest resilience by providing growing space to individual trees (Fettig et al. 2007; Hood et al. 2016; Knapp et al. 2021) but its impact on the dynamics of I. typographus remains unknown. Forest structures vary across elevation, slope, and aspect gradients, with elevation playing a crucial role in tree mortality during all phases of I. typographus infestation (Mezei et al. 2014a; Sproull et al. 2015). For example, trees tend to be more densely packed at lower elevations within a limited altitude range (Mazón et al. 2020; Zabihi et al. 2023). This difference in tree density due to elevation also affects tree characteristics like bark temperature and diameter (Fig. 2; Zabihi et al. 2023) which are important predictors of host susceptibility to bark beetles. Since topography and inter-tree spacing significantly modulate ecosystem functions, forest management strategies should be tailored considering these variables to foster improved overall health and resilience of secondary spruce forests (Zabihi et al. 2023; Thomas et al. 2024).

(Adapted from Zabihi et al. 2023)
A representation showing how variations in landscape, stand, and tree characteristics interact at different scales of elevation, tree density, and tree traits. The five circles in the diagram represent tree density gradients, with darker grey indicating higher density and lighter grey indicating lower density. The blue circle represents changes in elevation, with darker blue indicating higher altitude and lighter blue indicating lower altitude. The individual tree traits, such as bark temperature and diameter at breast height, shown in green color gradients, were found to be positively and negatively related to tree density, respectively.
4 Impact of drought stress on population dynamics of I. typographus
The intense and recurring drought events compromise the host tree defense mechanisms, rendering them more susceptible to infestation by bark beetles (Berryman 1972; Christiansen et al. 1987; Erbilgin et al. 2021). Research indicates that severe drought events limit the production of defense metabolites, unlike moderate drought, which is hypothesized to limit tree growth but enhance carbon allocation to defenses (Desprez-Loustau et al. 2006; Ferrenberg et al. 2015). Drought stress occurs when the available water in the soil falls below a specific threshold, leading to decreased soil water content and increased hydraulic resistance at the root-soil interface. Water transfer through xylem tissues may become irreversibly disrupted due to water cohesion breakdown and vessel embolism, increasing the risk of premature mortality of roots and twigs (Cruiziat et al. 2002; Cochard et al. 2009).
Isohydric conifer species avoid or delay the decline in xylem water potentials by restricting transpiration through stomatal closure (Rothe et al. 2002; Schume et al. 2004). Although stomatal regulation helps in maintaining water potential in the xylem, reduction in sap flow and leaf-atmosphere gas exchange declines the photosynthetic product assimilation regardless of continued respiratory consumption of carbon (Bréda et al. 2006; Rennenberg et al. 2006). Eventually, stored carbon reserves (i.e., non-structural carbohydrates) are depleted by repeated bark beetle attacks, and due to no or low replenishment as trees cannot sustain the production of defense metabolites (Erbilgin et al. 2021). At the same time, while tighter stomatal regulation can help avoid a decline in lethal xylem water potentials, the tension in the water conduits may cause shrinkage in tracheid diameters. This, together with a decrease in turgor pressure inside the epithelial cells lining the resin ducts, reduces the physical pressure on the oleoresin and subsequently reduces the exudation rate upon wounding of the bark, which may compromise the tree’s defense against frost, another drought episode and pest attacks, ultimately leading to tree mortality (Cruiziat et al. 2002; Bréda et al. 2006; Cochard et al. 2009; Rissanen et al. 2016).
Despite the well-known negative impacts of drought events on tree physiology and inherent defensive mechanisms, there is no conclusive evidence connecting drought-induced physiological stress and the tree’s attractiveness to bark beetles (Netherer et al. 2014). The Rosalia Roof experiment on drought manipulation addresses the issue of resource distribution in Norway spruce, focusing mainly on secondary metabolism (Netherer et al. 2014, 2019; Matthews et al. 2018). The project aimed to determine the impact of drought stress on both inherent and triggered defense mechanisms of trees against bark beetle attacks, including factors like resin flow and hypersensitive reactions to blue stain fungus inoculation. Furthermore, a recent field study has shown that when bark beetles infest drought-stressed trees, it not only decreases the local availability of carbohydrates crucial for essential tree functions but also hinders the tree’s capacity to replenish carbohydrate reserves (Erbilgin et al. 2021). Nevertheless, further research is needed to determine whether there is a difference in the attractiveness of stressed and control trees and validate the hypothesis that susceptible host trees are more attractive to pioneer beetles (Wermelinger 2004; Netherer et al. 2014).
5 Changing temperature regimes and their impact on population dynamics of I. typographus
Bark beetle outbreaks have been correlated with shifts in temperature and precipitation regimes as host tree vigor is affected by warmer spring and summer temperatures combined with increased water stress (Powell and Logan 2005; Berg et al. 2006). Bark beetle population growth and survival depend on thermal conditions as it can shorten development time and increase the number of generations per year (Bentz et al. 2010; Marini et al. 2012). Moreover, winter mortality is another critical component in bark beetle population dynamics (Hinze and John 2020). While the cold temperature adaptations and cold hardening mechanisms of many bark beetle species remain relatively unexplored, some Ips and Dendroctonus beetles stand out for their ability to accumulate cryoprotectant compounds (e.g., glycerol) during the colder periods of autumn, resulting in the decreased mortality (Lombardero et al. 2000; Bentz et al. 2010; Koštál et al. 2011). Most bark beetle species have symbiotic relationships with microorganisms like blue-stain fungi and bacteria that increase their tolerance to cold temperatures and provide nutrients to the larvae (Bleiker and Six 2007; Ayres et al. 2000; Guevara-Rozo et al. 2020).
The ambient temperature also influences the flight activities of bark beetles. The optimal flying activity occurs from 22 to 26 °C, while I. typographus do not swarm below 16.5 °C (Hinze and John 2020). Swarming of beetles in search of suitable breeding material is affected by two factors: the emergence from overwintering, which can be anticipated by thermal sum, and the mass flight of beetles, which occurs at a temperature above 20 °C (Annila 1969; Wermelinger 2004). A recent study showed that most I. typographus were caught on the hottest day (maximum temperature of 33.4 °C) of the observation period (mean air temperature 19.2 °C), suggesting that its ability to find hosts and mass flight is not compromised by increased thermal conditions (Hinze and John 2020). Furthermore, the average flight distance of I. typographus increases significantly on days with moderate temperatures than cold temperatures (Wermelinger 2004; Hinze and John 2020). Due to the rapid genetic adaptation of insects to seasonal changes in temperature regimes, range expansion of bark beetles beyond their habitat has also been observed where species move into new niches facilitated by increasing temperature (Balanyá et al. 2006; Battisti et al. 2006; Bradshaw and Holzapfel 2006; Nealis and Peter 2008; Erbilgin et al. 2014; Erbilgin 2019). Bark beetle dispersal-related ecological and environmental factors are reviewed in detail by Jones et al. (2019).
Regional-scale flight activity periods of I. typographus have been established by analysing climate data, focusing on temperature parameters. For instance, in southern Sweden, the onset of spring flight among I. typographus occurred when the accumulated thermal sum, averaging above 5 °C, persisted for roughly 47 ± 24 days (Öhrn et al. 2014). This period aligns with the findings from Denmark (Harding and Ravn 1983) and southern Finland (Annila 1969; Öhrn et al. 2014), where a similar flight duration of 45 days was observed. The flight period in Denmark lasted from early May to mid-August, whereas, in central Europe, it occurred between April and September (Faccoli and Stergulc 2004; Baier et al. 2007; Öhrn et al. 2014). In the region of southern Sweden, the flight activity period of I. typographus was observed to take place from mid-April to mid-August, which can be attributed to the effects of climate change resulting in warmer spring and summer temperatures compared to three decades ago (Öhrn 2012; Öhrn et al. 2014). In central European forests, sister broods of I. typographus with a minimum of two subsequent generations have been documented during the summer (June to August). Consequently, these temperature conditions have led to an increased frequency and extension of the flight activity periods of bark beetles (Netherer et al. 2019).
6 The current and potential future management practices
Bark beetle management strategies involve a range of approaches and typically start with removing windthrown and trees recently infested by Ips typographus. Anti-attractants or trap trees are commonly used to control beetle populations, whereas methods like remote sensing and canine detection are employed for early detection and mitigating attack damage. Furthermore, modern molecular tools are being investigated for controlling forest pests. In this section, we will discuss the traditionally adopted measures (salvage and sanitation harvesting, pheromone or trap-tree, short rotation forestry), progressive-practical methods (remote sensing, detection dogs) and explore promising cutting-edge molecular methods (Sterile insect technique (SIT), clustered regulatory interspaced short palindromic repeats/cas9 (CRISPR/Cas9) and RNA interference (RNAi)).
6.1 Reduction of rotation periods
Norway spruce forests often have rotation periods exceeding 100 years in many regions of Europe, resulting in extensive forest areas that are particularly prone to wind and bark beetle disturbances. For example, in Slovakia, between 1998 and 2009, less than a quarter of Norway spruce could reach 100 years of age (Hlásny et al. 2017). Because of these vulnerabilities, reducing the duration of rotation can be an effective strategy for forests to adapt to the growing bark beetle pressures (Zimová et al. 2020). Given that the optimal rotation period varies depending on the forest management system, site productivity, and species mixture (Hlásny et al. 2017), forest managers should consider the length of the current rotation period based on regional conditions. However, reducing the rotation period may also result in a decline in biodiversity and the amount of carbon stored in forests.
6.2 Optimizing forest composition to enhance resilience against bark beetles
Due to rapid population and economic growth, forests are under increasing pressure to meet demands for wood and associated products (Liu et al. 2018). Consequently, extensive areas across the globe are being cleared and transformed into plantation forests to cater to this demand (West 2014). Plantation forests vary in their intended purposes, and different species are chosen accordingly. Monocultures emerged in Europe during the eighteenth and nineteenth centuries as a response to timber scarcity, aiming to achieve high quality products (Griess and Knoke 2011). For instance, Norway spruce trees were extensively harvested in most parts of Europe due to their rapid growth rate and favourable timber characteristics (Spiecker 2000; Caudullo et al. 2016). However, frequent drought events are threatening the harvesting plantations. For instance, the significant increase in spruce mortality in Europe from 1970 to 2019 was partly attributed to the frequent drought events and heatwaves, with alterations in the structure and composition of forests accounting for approximately half of the documented increase in bark beetle outbreaks (Seidl et al. 2011). Furthermore, ecosystem services such as carbon sequestration, water regulation, and habitat provision are compromised in monocultures (Barrette et al. 2023) because a higher number of species with diverse functional traits can collectively contribute to a wide range of ecological functions (Lefcheck et al. 2015). Apart from the deliberate cultivation of Norway spruce beyond its native range, the augmentation of growing stocks and alterations in age-class distributions have significantly enhanced the susceptibility of spruce forests to Ips typographus attacks (Seidl et al. 2011; Hlásny et al. 2021).
In response to diebacks of monoculture forests, increasing tree diversity and age classes has been tested and recommended to increase forest resilience against biotic and abiotic disturbances (Lohbeck et al. 2016; Singh et al. 2023). In fact, there is a large number of empirical evidence suggesting that planting multiple species can provide several environmental, economic, and social benefits, and careful selection of species in mixed plantations can facilitate the promotion of complementary structural and functional traits (Hartley 2002; Forrester et al. 2005; Pawson et al. 2013; Carnol et al. 2014; Alem et al. 2015; Drössler et al. 2015). Thus, adopting mixed plantations as a strategy is more favourable than monocultures (Zhang et al. 2022). For instance, Dedrick et al. (2007) evaluated risks for several forest types and found that monoculture Norway spruce forests were more susceptible to biotic and abiotic disturbances than mixed-species forests. Including trees with varying age classes in mixed forests offers the potential for improved canopy coverage, leading to reduced solar radiation reaching the forest floor, which may minimize ground-level evaporation (Singh et al. 2023).
The argument that fostering tree species diversity lowers the risk of bark beetle infestation (Klapwijk et al. 2016) can be attributed to two main factors: Firstly, as discussed, mixed forests provide greater resilience against windthrow and storm damage; both of which are crucial drivers of the initiation of bark beetle outbreaks. In addition, an abundance of diverse tree species fosters various natural enemies of bark beetles including predators, pathogens, and parasitoids (Jactel and Brockerhoff 2007; Klapwijk and Björkman 2018; Stemmelen et al. 2022) suppressing the bark beetle population. Secondly, bark beetles rely on several cues for host selection, including olfactory, gustatory, and visual (Campbell and Borden 2006), and these cues help beetles to discriminate, for instance, defensively compromised trees (Rodriguez and Redman 2008; Schiebe et al. 2019). However, such cues may be masked by diverse volatile emissions from non-host trees in mixed forests during the host selection (Schiebe et al. 2011). Furthermore, tree diversity benefits the host tree species preferred by bark beetles; as such trees benefit from being hidden among non-host trees in the stand (Berthelot et al. 2021). However, tree species richness only works to suppress bark beetle outbreaks and not to avoid them, and even when the proportion of host plants is below 40% in the stand (de Groot et al. 2023). These facts demonstrate the importance of species richness in the context of bark beetle infestation. Yet, more evidence from field studies involving choice bioassays is needed to determine the effects of non-host volatiles on beetle infestation dynamics.
6.3 Early detection of infested trees, monitoring, and mass trapping
6.3.1 Detection dogs
Successfully implementing a management strategy that relies on rapid detection of bark beetle infestations and removing recently infested trees is necessary for forest protection. However, human detection generally requires close inspection (≤ 1 m) of trees and is therefore time-consuming, costly, and not always possible (Svensson 2007). Thus, aerial detection of infested trees generally occurs 2–3 months after an infestation, when tree crown colour fades, and bark falls off. At this point, the majority of bark beetles have already left infested trees and might target other uninfested trees. Using trained detection dogs has proven an effective alternative to locating infested trees (Johansson et al. 2019). The main advantages of using trained detection dogs in finding infested trees are their incredible sense of smell and capacity to explore large areas quickly (Hepper and Wells 2015; Mosconi et al. 2017). Vošvrdová et al. (2023) reported that trained sniffer dogs can locate infested trees up to 150 m away. Thus, such dogs can extend the time window for finding and removing infested trees, potentially avoiding the growth of larger infestations. As a limitation, detection dogs cannot be used for larger and remote areas.
6.3.2 Pheromone traps
Various trapping techniques have been widely used in bark beetle management in addition to sanitation felling and removal of windthrown trees. The rationale for using pheromone traps is to monitor beetle populations or reduce beetle numbers below the outbreak density by mass-trapping. The approach uses pheromone trap barriers, which aim to reduce the bark beetle population to a level where trees can successfully defend themselves against attacks (Jakuš 1998, 2001). Trapping techniques include using trap trees or log traps baited with species-specific pheromones or host compounds that may attract beetles. Once attracted, beetles can be killed with pesticides or by removing the infested trees or logs. Limited scientific evidence supports the efficacy of using trap trees to effectively reduce beetle populations or the number of attacked trees (Klutsch et al. 2017). However, there is a shortage of research on the efficacy of trapping techniques in reducing the risks of outbreaks and rates of damage, particularly for large-scale applications. While pheromone traps typically capture only 3–10% of beetle populations at a relatively high cost (Wermelinger 2004), using pheromone trap barriers as part of the integrated forest protection system makes it possible to reduce tree mortality significantly (Jakuš 1998, 2001). Due to relatively high labour costs, using pheromone traps has been discontinued in Scandinavia and most parts of Germany and France. The use of pheromone traps for mass trapping of bark beetles is further constrained by the “spillover effect,” where commercial pheromones attract more beetles than the traps could handle, causing attacks in trees adjacent to the traps (Niemeyer 1997; Jakuš et al. 2022). Nevertheless, the primary purpose of such traps should continue to monitor the bark beetle population rather than population reduction and outbreak suppression. Further improvement in using pheromone trap barriers is possible with the new mixtures of attractants (Blaženec et al. 2021; Jirošová et al. 2022).
6.3.3 Anti-attractants
Anti-attractants are semiochemicals used to disrupt the host-finding behavior of pests. By emitting repelling signals to pests or masking the attractive signals emitted by potential hosts, anti-attractants can protect trees from being located and colonized by these pests. Among the range of active anti-attractant compounds identified for I. typographus (Schiebe et al. 2011), the first notable one is verbenone, generated from the host compound α-pinene or converted from I. typographus’s main pheromone component cis-verbenol (Birgersson and Leufvén 1988). The second group comprises non-host volatiles (NHVs): trans-conophthorin, an important synergistic compound found in the bark of broad-leaf trees (Zhang and Schlyter 2003); green leaf volatiles (GLV; 1-hexanol; (Z)-3-hexen-1- ol; I-2-hexen-1-ol), detected in non-host birch (Betula spp.) and aspen (Populus tremula) (Zhang et al. 1999); and C8 alcohols (3-octanol; 1-octen-3-ol), emitted from the bark of the mentioned species. Attempts to protect logs or fallen trees with anti-attractants have not been successful in stopping bark beetle attacks, including I. typographus (Jakuš and Blaženec 2003).
Currently, the most promising methods for defending standing spruce trees against I. typographus attacks involve dispensers with a blend of verbenone and NHV compounds (Schiebe et al. 2019). A recent study reported a strong “switching effect,” of using anti-attractants, which involves pushing away beetles from areas with anti-attractants into areas without non-attractants (Jakuš et al. 2022). Furthermore, installing anti-attractant dispensers at two different heights on trees showed no difference, suggesting that using anti-attractants is ineffective in areas affected by severe drought and extremely high bark beetle populations (Jakuš et al. 2022). Unusually, ( +)-trans-4-thujanol repelled female I. typographus, demonstrating its efficacy to be on par with known anti-attractants such as 1,8-cineole and verbenone, making it an innovative anti-attractant for forest protection (Jirošová et al. 2022). A recent meta-analysis by Afzal et al. (2024) on the push–pull strategy, which involves using attractive and repellent semiochemicals, reports that this strategy can reduce Ips and Dendroctonus populations by 66% and 54%, respectively, compared to the control.
6.3.4 Remote sensing (RS) of forests
Gathering precise and current spatial information on the presence and dynamics of the bark beetle infestation is still challenging in large areas with limited access (Stereńczak et al. 2019). Recently, several research teams in Europe and North America have focused on studying the spatio-temporal analyses of bark beetle population dynamics (Simard et al. 2012; Kärvemo et al. 2014; Meddens and Hicke 2014; Senf et al. 2015; Havašová et al. 2017; Mezei et al. 2017; Marvasti-Zadeh et al. 2024). Forest managers will likely need to face widespread and frequent infestations of bark beetles in the near future, necessitating the need to develop efficient tools that can assess the current spread and dynamics of insect outbreaks in a given area rapidly and precisely. Precise and up-to-date spatial information on bark beetle outbreaks (e.g., locations of dead trees) is essential when planning protective and sanitary actions (e.g., pheromone or tree trapping) to control or limit the intensity of an outbreak in a given area (Fassnacht et al. 2014; Fettig and Hilszczański 2015). The ability to identify and map tree mortality caused by bark beetle outbreaks depends mainly on the forest structure. For example, detecting and monitoring forest stands that consist solely of coniferous host species is relatively straightforward. New dead trees in such stands mostly appear in large, easily detectable groups as bark beetles primarily attack trees adjacent to those already killed (Lausch et al. 2011; Seidel et al. 2016). Detection of individual-infested trees can change in complex forest structures consisting of mosaics of various stands with mixed tree species where the typical host tree species occur in smaller scattered groups or individual trees. Identifying individual infested trees in such a situation is a challenging task, and as financial resources typically limit fieldwork, RS methods are an effective alternative and supplement to field surveys. Multispectral aerial and satellite imagery, in combination with artificial intelligence and machine learning, has been successfully used for mapping insect outbreaks and other forest disturbances (Roberts et al. 2003; White et al. 2007; Long and Lawrence 2016; Senf et al. 2017; Marvasti-Zadeh et al. 2024). For instance, the significant spectral differences between the healthy and stressed trees (i.e., drought) were found on imageries of the Enhanced Vegetation Index (EVI) and Visible Atmospherically Resistant Index (VARI) at the beginning of the growing season before the new attacks. The results highlight the potential of using SVIs derived from high-resolution multispectral imagery to detect pest infestations early and manage forest ecosystems (Trubin et al. 2023, 2024).
We could rely on various RS data and sources to estimate tree health characteristics. Remote sensing generally uses satellite- or aircraft-based (e.g., Unmanned Aerial Vehicle) sensor technologies. In forestry, especially in the case of examination of the spectral properties of trees, we can use active (such as LIDAR) and passive sensors (such as Muti- and Hyperspectral sensors) (Niemann et al. 2015). The most recent methods in using, processing, and analysing RS in the context of tree predisposition determination combine sensor data and represent multi-temporal GIS analysis based on hyperspectral and airborne laser scanning data (Abdullah et al. 2019; Stereńczak et al. 2019). Hyperspectral and airborne laser scanning data are widely used to identify prominent forest disturbances in the bark beetle outbreak sites and dead trees in the red and grey attack stages. The change in foliage colour in trees affected by infestation becomes noticeable after approximately 6 to 8 months from the onset of the infestation. This transformation in the crown of infested trees from green to yellow to red is due to the gradual loss of moisture and the deterioration of pigments within the foliage. These distinct stages in the colour change are referred to as the “red-attack” and “grey-attack” phases, respectively (Safranyik and Carroll 2007). Analysis of red and grey attack data allows us to understand the dynamics of the bark beetle outbreak, and the accumulation of additional data, such as climatic (temperature, wind speed, precipitation), will allow us to understand the most critical drivers of the outbreak.
The possibility of identifying all attacked trees is limited by the output data available for analysis from visible dominant trees in the first forest layer. Another associated challenge is incorrectly identifying dead trees as living due to reflection from the lower layer (Stereńczak et al. 2019). Similarly, early detection of trees attacked by bark beetles depends on the availability of ground truth data (Nardi et al. 2023; Trubin 2024; Kautz et al. 2024). However, acquiring ground truth data is often labour-intensive (Zabihi et al. 2021) and only limited to areas accessible by the field crew. Additionally, early attacked trees are challenging to identify using remote sensing when there are stressed trees around and due to changes in the foliar colour of spruce trees at the beginning of the season. Another limitation relates to the limited computational power for analysing large-scale data.
6.4 Biological control agents
6.4.1 Natural enemies
Biological control involves suppressing a pest by introducing a naturally occurring antagonist to achieve sustainable and eco-friendly control over insect pests (Kenis et al. 2017). Natural enemies such as predators, parasites and parasitoids impact bark beetles’ population dynamics and ecology. The variety of antagonists that target bark beetles is extensive and includes the order Hymenoptera, beetles (Coleoptera), flies (Diptera), true bugs (Heteroptera), snake flies (Raphidioptera), and mites (Acari) (Table 1).
Bark beetle predators are highly diverse, and can consume all life stages of bark beetles, from eggs to adults, and significantly influence their population dynamics. Although numerous predator species are associated with bark beetle galleries, only a few actively prey on eggs, larvae, pupae, and/or adults. Most predators targeting bark beetles belong to Coleoptera (beetles), including Cleridae, Trogossitidae, and Rhizophagidae (Hopping 1947; Mills 1985; Wermelinger 2002). The most notable coleopteran predators of bark beetles are the checkered beetles (Cleridae). This small family includes several early-season predatory species, such as Thanasimus dubius (F.) from North America and Thanasimus formicarius (L.) from Europe (Stephen and Dahlsten 1976; Herard and Mercadier 1996; Lawson et al. 1997). Clerid beetles arrive at the infested trees shortly after bark beetles and consume the beetles directly on the bark surface. Thanasimus formicarius is among the most studied predators of I. typographus (Kenis et al. 2007). Its females lay approximately 100 eggs, and adults can consume up to three beetles per day, while larvae can consume approximately 50 bark beetle larvae throughout their larval development stages (Mills 1985; Dippel et al. 1997). While clerid beetles are typically considered generalist predators, trogossitids are believed to have a more specialized approach (Kohnle and Vite 1984; Lawson and Morgan 1992). For instance, Temnochila virescens primarily responds to attractants produced by Ips species, and their larvae feed on bark beetle larvae and pupae within the tree’s phloem, whereas adult Temnochila species consume the adults of various other bark beetles (Billings and Cameron 1984).
Furthermore, parasitoids can be an important part of the population dynamics of bark beetles. The parasitoids prefer a specific developmental stage of the host. Most parasitoids associated with Scolytids belong to the Hymenoptera order, including Braconidae and Pteromalidae. They can attack various developmental stages of bark beetles. Each parasitoid larva typically consumes one beetle larva or pupa. The most effective parasitoid of I. typographus is Coeliodes bostrichorum, and it appears to be exclusively associated with bark beetle species breeding in Norway spruce (Feicht 2006; Kenis et al. 2007).
Various organisms, including wasps, ants, birds, shrews, mites, and pathogens like viruses and microsporidia are potential antagonists of I. typographus, but their impact on population dynamics requires further understanding (Kenis et al. 2007). Muratoğlu et al. (2011) identified five pathogenic bacterial genera associated with I. typographus mortality, with Serratia liquefaciens showing notable effectiveness, causing 53.3% mortality. Commercially produced entomopathogenic microbes, including fungal species like Beauveria bassiana, Metarhizium anisopliae, Hirsutella guignardii, Isaria farinosa, and Lecanicillium lecanii have been utilized as microbial control agents against Ips and Dendroctonus (Kreutz et al. 2004; Popa et al. 2012; Lacey et al. 2015; Mann and Davis 2021; Rosana et al. 2021; Fernandez et al. 2023). Entomopathogens serve as effective pest controllers, efficiently managing insect populations while also being environmentally friendly towards non-target organisms (Hajek and Bauer 2009). However, despite their potential, challenges such as susceptibility to ultraviolet light, low moisture, temperature fluctuations, plant secondary metabolites, and competition with other microorganisms limit the widespread field application (Mann and Davis 2021).
While biological control strategies offer innovative and eco-friendly methods for bark beetle management, only a few biological control strategies against bark beetles have been implemented so far. The bark beetles are often attacked by polyphagous enemies, which typically have minimal impact on regulating the beetle populations (Kenis et al. 2007). In most cases, the lack of specificity of many natural enemies of bark beetles would make them unsuitable as biological controls, except the successful use of the specific predatory beetle Rhizophagus grandis to effectively control the great spruce bark beetle in areas like the Caucasus and Western Europe (Grégoire et al. 1992; Averbeke and Grégoire 1995). Apart from that, biological control programs also struggle to prove their financial benefits. Politically, this impedes government investment in biological control research and development and reduces academic interest in further exploration. At the management level, lack of engagement leads to skepticism about the effectiveness and financial advantages compared to pesticides (Barratt et al. 2018). Understanding ecological and environmental factors (i.e., bark beetle attack dynamics, predator population and their co-existences, the impact of changing climates on prey and predators, and the effect of other pesticide-based control measures on natural enemies) determining the efficiency of biological control methods against bark beetles need to be thoroughly investigated for successful incorporation of natural enemies in forest pest management programs.
6.4.2 Sterile insect technique (SIT)
The sterile insect technique involves dispersing a large number of sterile male insects into the population in a given area, with the expectation that these males mate with normal females, resulting in non-viable offsprings and lower the population densities of pests in the generation that follows (Lance and McInnis 2005; Diallo et al. 2019; Tam et al. 2023). Both sterile sexes can be released when it is impossible to distinguish between the sexes, making the procedure more successful while helping to keep the pest population below the epidemic threshold (Hendrichs et al. 2021; Ikegawa et al. 2021). The SIT relies on the irradiation of insects by gamma radiation from isotopic elements (cobalt-60 or cesium-137) as well as high-energy electrons and X-rays. To effectively sterilize the insect’s reproductive cells while still keeping them alive enough to compete for mating, the radiation dose must be tightly managed because radiation can harm the sex cells of insects by causing chromosome fragmentation (dominant lethal mutation, translocations, and/or chromosomal aberrations), which results in the production of unbalanced gametes. Hence, mitosis is inhibited, and fertilized egg/embryo development is impaired (Bakri et al. 2021; Klassen and Vreysen 2021; Robinson 2021). The lethality of the dose–effect on two lepidopteran pests (Ostrinia nubilalis and Phyllocnistis citrella) indicates that higher doses of gamma radiation reduce the life span of these insects in both sexes. However, the larval emergence of the F1 was skewed towards males. The effectiveness of this technique is studied and confirmed in agricultural insect pests like sweet potato weevil (Cylas formicarius), pepper weevil (Anthonomus eugenii), and I. typographus (Turčáni and Vakula 2007; Čičková et al. 2018; Ikegawa et al. 2022; Basso et al. 2023). While SIT proves to be an economical, sustainable, and efficacious approach, its potential for extensive implementation in large-scale field scenarios has not been examined and continues to pose a constraint.
6.5 Genetic approaches
6.5.1 Utilizing genetic information of host-tree
The degree and frequency of drought events have recently increased for forests worldwide due to rapid climate change, and adaptation of trees to the new disturbance regimes can be locally scaled at the gene level and includes substantial selection pressure on tolerant phenotypes (Zacharias et al. 2022). Norway spruce exhibits higher lignin synthesis and increased expression of defense-related genes in response to shade, potentially enhancing its resilience against pest attacks (Ranade et al. 2019, 2022). Tree responses to heat stress are influenced by signaling factors (protein kinases and transcription factors), heat shock proteins, heat stress factors, and catalase enzymes. In contrast, ascorbate peroxidase and histidine kinases remove reactive oxygen species during heat stress, while dehydration-responsive element-binding proteins shield trees from osmotic stress.
According to spatial and differential gene expression examination of conifers, stomatal closure and cuticular wax on the surface of needles can reduce water loss. A study on needles of maritime pine (Pinus pinaster) and Norway spruce reported that in non-irrigated saplings, the cuticular wax and the genes involved in its synthesis were overexpressed as compared to the irrigated ones (Blödner et al. 2007; Le Provost et al. 2013). With the advancement of molecular techniques and high throughput sequencing methods, the use of molecular tools to target genes for pest management is highly popularized (Singh et al. 2024). Various genes have been employed in agricultural pest management since the cry gene was identified in the 1980s, which was among the initial unique genes utilized for GM crops to combat insect pests. These advancements, however, are still in their early stages and are constrained by factors like tree size, density, and application at the forest stand level. Hence, additional technologies were diverted to insect-level genetic modifications, which led to the development of various new techniques such as sterile insect technique, gene silencing via RNAi and CRISPR, and other pre- and post-translation level modifications. Identifying genetic markers of tree resistance can be applied to forest protection, tree health diagnosis, and breeding bark beetle-resistant trees (Korecký et al. 2023).
6.5.2 RNA interference (RNAi)
In insects, RNAi is a conserved cellular process that turns off gene function by interfering with mRNA breakdown and protein production (Fire et al. 1998; Zhu and Palli 2020; Mogilicherla and Roy 2023a). This process involves silencing specific genes by using short RNA molecules to target and degrade messenger RNA (mRNA), preventing the production of proteins encoded by those genes. The silencing or “turning off” of vital genes for the survival of insects ultimately results in mortality (Zhu and Palli 2020).
Three distinct RNAi mechanisms have been identified: small interfering RNA (siRNA), microRNA (miRNA), and piwiRNA (piRNA). The siRNA pathway has received the most attention in insect pest management (Zhu and Palli 2020). In a nutshell, the siRNA machinery is activated by the successful delivery of double-stranded RNA (dsRNA) into the cell, then Dicer-2 (ribonuclease type III) enzyme converts the dsRNA into siRNAs (~ 21–24 bp), which are subsequently integrated into the RNA-induced silencing complex. Eventually, Argonaute2 cleaves and removes the sense strand of the siRNA. The remaining antisense strand then directs the RNA-induced silencing complex to sequence-specific targeting of complementary mRNA strand, which leads to degradation of the mRNA strand and post-transcriptional gene silencing (Zhu and Palli 2020). This sequence-specific mechanism of RNAi can be harnessed to effectively target vital genes in insects, including bark beetles, offering a means of pest management (Zhu and Palli 2020; Joga et al. 2016, 2021; Mogilicherla and Roy 2023b).
Researchers have developed RNAi-biopesticides that effectively silenced the target genes and caused decent mortality in beetles such as the Colorado potato beetle (Leptinotarsa decemlineata), emerald ash borer (Agrilus planipennis), Asian long-horned beetle (Anoplophora glabripennis), mountain pine beetle (Dendroctonus ponderosae Hopkins) and southern pine beetle (Dendroctonus frontalis) (Table 1) (Yoon et al. 2016, 2018; Rodrigues et al. 2017a, 2017b; Máximo et al. 2020; Dhandapani et al. 2020a, 2020b; Kyre et al. 2019, 2020; Kyre and Rieske 2022). The putative variability of RNAi among genetically variable beetles in geographically distinct populations was also recently documented (Kyre et al. 2024). Recent research has led to the development of the RNAi-based biopesticide Ledprona against L. decemlineata, which prevents the expression of enzymes, promotes protein breakdown, and ultimately results in mortality (Pallis et al. 2023). RNAi provides excellent promise for minimal environmental impact, attributed to its precise targeting and the transient nature of its active molecules. However, additional research is needed before widespread use of such commercial products in forestry. To ensure its safety, a combination of bioinformatics and ecologically sound bioassays with selected focal insect species will help a thorough understanding of potential off-target effects and impacts on non-target organisms (Christiaens et al. 2022; Mezzetti et al. 2022). These products must be used only to maintain the bark beetle populations under endemic conditions and preserve the beneficial role of bark beetles as decomposers in forest ecosystems.
Bark beetles are more susceptible to RNAi, but the effectiveness depends on the target gene selection, dsRNA stability, and expression of the RNAi core machinery genes (Mogilicherla et al. unpublished data). Effectiveness, off-target and non-target effects, and a lack of reliable dsRNA delivery mechanisms are the main obstacles to the widespread use of RNAi for bark beetle pest management. In contrast, feeding techniques combined with advanced development technologies (symbiont-mediated and nanoparticle-enabled) are critical for improved dsRNA transport, stability, endosomal escape, and dsRNA processing (Joga et al. 2021; Mogilicherla and Roy 2023b). Recently, Mogilicherla and Roy (2023b) comprehensively reviewed chitosan-dsRNA nanopesticides and their applications in managing bark beetles. The bacteria and fungi that live symbiotically with bark beetles have been successfully isolated and identified and can be considered putative candidates for symbiont-mediated RNAi (SMT) for tropical application to control bark beetles (Chakraborty et al. 2020a, 2020b, 2023; Gupta et al. 2023; Mogilicherla and Roy 2023b). These studies may pave the way for developing and using RNAi-biopesticides as a secure, efficient, and innovative method to safeguard forest trees. Fortunately, current research conducted globally and recent work on the genome, transcriptome, and proteome of bark beetles and their symbiotic microbes will significantly enhance the information on these insects and facilitate the development of species-specific RNAi-based biopesticides in the future (Powell et al. 2021; Ashraf et al. 2023; Naseer et al. 2023; Sellamuthu et al. 2023). Nonetheless, only a limited number of RNAi-based insecticides have obtained licensing and are on the verge of becoming accessible in the market (Li et al. 2023; Pallis et al. 2023).
6.5.3 CRISPR/Cas9
Numerous studies have made considerable use of the ground-breaking genome editing tool known as clustered regulatory interspaced short palindromic repeats/cas9 (CRISPR/Cas9) (Sun et al. 2017; Singh et al. 2022; Yan et al. 2023). CRISPR/Cas9 is a gene editing technology used to alter the DNA of organisms. In the CRISPR/Cas system, CRISPR comprises DNA sequences found in prokaryotic organisms like bacteria and archaea, while Cas9 is an enzyme that uses CRISPR sequences as a guide to locate and open specific DNA strands. The Cas9 enzyme attaches to the target DNA and cleaves it, deactivating the targeted gene. This process, known as gene “knock-out,” is a reliable method for identifying genes of interest or gaining deeper insights into genome complexities (Doudna and Charpentier 2014; Jiang and Doudna 2017).
CRISPR is a more intuitive and user-friendly technology because it needs only one guide RNA (gRNA) for target identification and Cas9 nuclease for implementation (Richter et al. 2013; Upadhyay 2021). The relevant derivative has a single chimeric guide RNA (sgRNA) that recognizes and binds to the intended target sequence, as well as a CRISPR-associated (Cas) nuclease that cleaves DNA at specific locations by producing site-specific double-strand breaks (Horvath and Barrangou 2010; Wiedenheft et al. 2012). The plasmid DNA, RNA, or ribonucleoprotein complex (RNPC) are some of the different delivery mechanisms that can be used to introduce the CRISPR/Cas components (sgRNA and Cas9 protein) into the target organism (Ogaugwu et al. 2013). CRISPR/Cas9 has an advantage over RNAi due to its ability to bring about enduring and inheritable genomic alterations. In contrast, RNAi only produces immediate effects unless a steady supply of dsRNA is kept available. Nevertheless, CRISPR technology has the potential to revolutionize pest control, but both the advantages and disadvantages must be carefully considered (Perkin et al. 2016). The CRISPR/Cas9 technology has been used against a wide variety of insect species; however, in the case of coleopteran insects, it has only been applied to the red flour beetle (Tribolium castaneum) and Colorado potato beetle (Leptinotarsa decemlineata) (Gilles et al. 2015; Gui et al. 2020; Singh et al. 2022).
6.5.4 Ethical and legal implications of RNAi and gene editing technologies in forest pest management
Development of regulatory protocols for modern biotechniques such as RNAi and CRISPR is essential before intended deployment. However, the regulatory protocols can vary across jurisdictions, predominantly emphasizing the process or the end product due to associated risks and the need to effectively manage toxicity levels to non-target organisms (Ahmad et al. 2021; Távora et al. 2022). The regulatory framework for genetically modified (GMO) products in the European Union and New Zealand is process-based and necessitates significant time and financial expenditure. In contrast, the end-product-based regulatory structure used by the US, Canada, China, and selected European countries is comparatively efficient in terms of time and cost. Before commercialization, the RNAi and gene-edited products must be checked for potential adverse effects on non-target organisms, including microorganisms in the habitat (i.e., forest soil). Therefore, discussing several regulatory regimes regarding RNAi and CRISPR systems in numerous jurisdictions worldwide is necessary.
Only a limited number of RNAi and CRISPR-based products have been approved for global commercial release, likely due to persistent regulatory obstacles and unsettling consumer perception and acceptance (Mat Jalaluddin et al. 2019). Besides, several important issues must be addressed before society accepts biotechnological products (Taning et al. 2021). Regular communication among researchers, foresters, and other pertinent participants (i.e., state forest enterprises and forest owners) is essential for reporting biotechnological breakthroughs. Furthermore, the contemporary acceptance of CRISPR and RNAi-based bioproducts, such as biotic stress-resistant plant varieties and biopesticides, depends on transparent communication referring to technical intricacies like gene editing and silencing mechanisms alongside a thorough exploration of potential risks and benefits. Scientists from relevant sectors are pivotal in facilitating open dialogue with forest organizations and sustaining educational efforts to foster informed decision-making (Rank and Koch 2021; Taning et al. 2021). Nevertheless, it is crucial to address ethical and moral considerations at the outset of developing CRISPR/Cas and RNAi-based technological solutions (Frewer et al. 2013; Gupta et al. 2015; Beghin and Gustafson 2021).
Interestingly, RNAi technique does not always require a transgenic expression of target dsRNA; the use of topically applied RNAi-based biopesticides or spray-induced gene silencing (SIGs) for the management of tree diseases may see a rise in public acceptance (Shew et al. 2017). Regulatory frameworks for topical RNAi-based products in agriculture or forestry are still nascent worldwide. Despite this, extensive research has been conducted into plant protection using topically applied RNAi-based bioproducts, driven by their promising benefits. Topical RNAi-based products offer clear advantages over most used crop protection methods using chemical pesticides. However, the environmental fate and stability of the naked dsRNA or dsRNA conjugates used in SIGs and their impact on beneficial microorganisms in the habitat must be assessed from case to case.
Although there is still much debate about the regulations surrounding CRISPR-edited organisms, a few nations have established regulatory frameworks designed to assess these products (reviewed by Devos et al. 2022). Some genetic engineering methods do not entail the introduction of exogenous DNA sequences. Such methodologies may not produce a final product classified as genetically modified (Podevin et al. 2013; Entine et al. 2021). In many countries, legislation governing GMOs or traditional chemical and biological pesticides does not cover these genetic engineering methods. Nevertheless, an approach based on scientific parameters to develop and validate biotechnological products is fundamental to formulating the most appropriate risk assessment protocols (Mezzetti et al. 2020).
7 Conclusions and future prospectives
A considerable proportion of global plantation forests consisting of monocultures, preferred for timber production owing to their uniformity and ease of management, are under threat. However, as extreme weather conditions like droughts and rising temperatures favour forest pest outbreaks, there is a pressing need for eco-friendly strategies to reduce the vulnerability of forests to climatic change. To enhance overall forest resilience, it is proposed that cultivating plantations with diverse species and promoting complementary traits could be a viable solution. However, despite being a longstanding priority on the political agenda in central Europe, realizing such goals has been slow. A recent report from the European Commission aims to promote a transition toward a sustainable and resilient silviculture practice by 2030 (European Commission 2021).
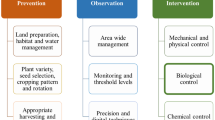
Understanding the mechanism of bark beetle infestation and outbreak dynamics at different spatial scales has serious implications for protecting and managing the Norway spruce ecosystem. Despite the ecological and economic impacts of bark beetle outbreaks, the current control measures are ineffective in stopping the spread of bark beetle outbreaks. Existing methods like pheromone and tree traps, anti-attractants, remote sensing, and canine detection of infested trees against bark beetles will continue to aid in managing bark beetles amidst climate change. However, additional approaches such as nanoparticle-coated RNAi complexes and symbiont-mediated RNAi, SIT, and CRISPR/Cas9-based approaches are needed and may potentially replace some of the current management approaches in combating bark beetles in forest management (Fig. 3). These new approaches have been very successful in controlling agricultural pests. They are relatively less explored for forest pest management, especially for bark beetles, for various reasons, including the availability of sequenced genomes, difficulties in rearing on an artificial diet, and obtaining viable eggs from beetles for injection. Regardless, extensive product development and testing their effectiveness in field studies are required before commercial manufacturing and widespread field application. In addition, the success of the potential application of the recent molecular techniques is contingent upon understanding their mechanisms, assessing potential risks, and navigating the global regulations governing similar genetic materials. It can be anticipated that molecular approaches will continue evolving in the research domain aimed at combating forest pests, and thus, the environmental safety of such approaches should be carefully monitored for safe deployment.
The Scheme illustrates the currently used traditional methods and prospective molecular techniques that can help manage bark beetles in weakened forest ecosystems. The upper panel shows the major abiotic factors that weaken the forest ecosystem and increase its susceptibility to bark beetle infestations. The traditional methods are helpful in monitoring and controlling bark beetle populations to some extent, but novel cutting-edge technologies can play a crucial role in eliminating pest outbreaks
Availability of data and material (data transparency)
Not applicable.
References
Abdullah H, Skidmore AK, Darvishzadeh R, Heurich M (2019) Sentinel-2 accurately maps green-attack stage of European spruce bark beetle (Ips typographus L.) compared with Landsat-8. Remote Sens Ecol Conserv 5:87–106. https://doi.org/10.1002/rse2.93
Afzal S, Nahrung HF, Lawson SA, Hayes RA (2024) How effective are push-pull semiochemicals as deterrents for bark beetles? A global meta-analysis of thirty years of research. InSects 14:812. https://doi.org/10.3390/insects14100812
Ahmad S, Shahzad R, Jamil S, Tabassum J, Chaudhary MA, Atif RM, Iqbal MM, Monsur MB, Lv Y, Sheng Z, Ju L (2021) Regulatory aspects, risk assessment, and toxicity associated with RNAi and CRISPR methods. In: CRISPR and RNAi Systems, Elsevier, pp 687–721. https://doi.org/10.1016/B978-0-12-821910-2.00013-8
Aldea J, Dahlgren J, Holmström E, Löf M (2023) Current and future drought vulnerability for three dominant boreal tree species. Glob Change Biol 30:e17079. https://doi.org/10.1111/gcb.17079
Alem S, Pavlis J, Urban J, Kucera J (2015) Pure and mixed plantations of Eucalyptus camaldulensis and Cupressus lusitanica: their growth interactions and effect on diversity and density of undergrowth woody plants in relation to light. Open J for 5:375–386. https://doi.org/10.4236/ojf.2015.54032
Annila E (1969) Influence of temperature upon the development and voltinism of Ips typographus L. (Coleoptera, Scolytidae). Ann Zool Fenn 6(2):161–208
Ashraf MZ, Mogilicherla K, Sellamuthu G, Siino V, Levander F, Roy A (2023) Comparative gut proteomics study revealing adaptive physiology of Eurasian spruce bark beetle, Ips typographus (Coleoptera: Scolytinae). Front Plant Sci. https://doi.org/10.3389/fpls.2023.1157455
Aukema BH, Raffa KF (2005) Selective manipulation of predators using pheromones: responses to frontalin and ipsdienol pheromone components of bark beetles in the Great Lakes region. Agric for Entomol 7:193–200. https://doi.org/10.1111/j.1461-9555.2005.00250.x
Aukema BH, Dahlsten DL, Raffa KF (2000a) Exploiting behavioral disparities among predators and prey to selectively remove pests: maximizing the ratio of bark beetles to predators removed during semiochemically based trap-out. Environ Entomol 29:651–660. https://doi.org/10.1603/0046-225X-29.3.651
Aukema BH, Dahlsten DL, Raffa KF (2000b) Improved population monitoring of bark beetles and predators by incorporating disparate behavioral responses to semiochemicals. Environ Entomol 29:618–629. https://doi.org/10.1603/0046-225X-29.3.618
Averbeke AV, Gregoire JC (1995) Establishment and spread of Rhizophagus grandis Gyll. (Coleoptera: Rhizophagidae) 6 years after release in the Forêt domaniale du Mézenc (France). Ann Sci for 52:243–250. https://doi.org/10.1051/forest:19950305
Ayres MP, Wilkens RT, Ruel JJ, Lombardero MJ, Vallery E (2000) Nitrogen budgets of phloem-feeding bark beetles with and without symbiotic fungi. Ecology 81:2198–2210. https://doi.org/10.1890/0012-9658(2000)081[2198:NBOPFB]2.0.CO;2
Baier P, Pennerstorfer J, Schopf A (2007) PHENIPS—a comprehensive phenology model of Ips typographus (L.) (Col., Scolytinae) as a tool for hazard rating of bark beetle infestation. For Eco Manag 249:171–186. https://doi.org/10.1016/j.foreco.2007.05.020
Bakri A, Mehta K, Lance DR, Dyck VA, Hendrichs J, Robinson AS (2021) Sterilizing insects with ionizing radiation. Sterile Insect Tech Princ Pract Area-Wide Integr Pest Manag. https://doi.org/10.1007/1-4020-4051-2_9
Balanyá J, Oller JM, Huey RB, Gilchrist GW, Serra L (2006) Global genetic change tracks global climate warming in Drosophila subobscura. Science 313:1773–1775. https://doi.org/10.1126/science.1131002
Barratt BI, Moran VC, Bigler F, Van Lenteren JC (2018) The status of biological control and recommendations for improving uptake for the future. Biocontrol 63:155–167. https://doi.org/10.1007/s10526-017-9831-y
Barrette J, Achim A, Auty D (2023) Impact of intensive forest management practices on wood quality from conifers: literature review and reflection on future challenges. Curr for Rep 9:101–130. https://doi.org/10.1007/s40725-023-00181-6
Basso JV, Labbe R, Scott-Dupree C (2023) Assessing the sterility and quality of gamma-irradiated pepper weevils, Anthonomus eugenii (Coleoptera: Curculionidae), toward the development of the sterile insect technique. Pest Manag Sci 80:1692–1701. https://doi.org/10.1002/ps.7898
Battisti A, Stastny M, Buffo E, Larsson S (2006) A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Glob Chang Biol 12:662–671. https://doi.org/10.1111/j.1365-2486.2006.01124.x
Bazzaz FA, Chiariello NR, Coley PD, Pitelka LF (1987) Allocating resources to reproduction and defense. Bioscience 37:58–67. https://doi.org/10.2307/1310178
Beghin JC, Gustafson CR (2021) Consumer valuation of and attitudes towards novel foods produced with new plant engineering techniques: a review. Sustainability 13:11348. https://doi.org/10.3390/su132011348
Bentz BJ, Rgnire J, Fettig CJ, Hansen EM, Hayes JL, Hicke JA, Kelsey RG, Negron JF, Seybold SJ (2010) Climate change and bark beetles of the western United States and Canada: direct and indirect effects. Bioscience 60:602–613. https://doi.org/10.1525/bio.2010.60.8.6
Berg EE, David Henry J, Fastie CL, De Volder AD, Matsuoka SM (2006) Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: relationship to summer temperatures and regional differences in disturbance regimes. For Ecol Manag 227:219–232. https://doi.org/10.1016/j.foreco.2006.02.038
Berryman AA (1972) Resistance of conifers to invasion by bark beetle-fungus associations. Bioscience 22:598–602. https://doi.org/10.2307/1296206
Berryman AA, Raffa KF, Millstein JA, Chr N (1989) Interaction dynamics of bark beetle aggregation and conifer defense rates. Oikos. https://doi.org/10.2307/3565345
Berthelot S, Frühbrodt T, Hajek P, Nock CA, Dormann CF, Bauhus J, Fründ J (2021) Tree diversity reduces the risk of bark beetle infestation for preferred conifer species, but increases the risk for less preferred hosts. J Ecol 109:2649–2661. https://doi.org/10.1111/1365-2745.13672
Biedermann PHW, Müller J, Grégoire JC, Gruppe A, Hagge J, Hammerbacher A, Hofstetter RW, Kandasamy D, Kolarik M, Kostovcik M, Krokene P, Sallé A, Six DL, Turrini T, Vanderpool D, Wingfield MJ, Bässler C (2019) Bark beetle population dynamics in the anthropocene: challenges and solutions. Trends Ecol Evol 34:914–924. https://doi.org/10.1016/j.tree.2019.06.002
Billings RF, Cameron RS (1984) Kairomonal responses of Coleoptera, Monochamus titillator (Cerambycidae), Thanasimus dubius (Cleridae), and Temnochila virescens (Trogositidae), to behavioral chemicals of southern pine bark beetles (Coleoptera: Scolytidae). Environ Entomol 13:1542–1548. https://doi.org/10.1093/ee/13.6.1542
Birgersson G, Leufvén A (1988) The influence of host tree response to Ips typographus and fungal attack on production of semiochemicals. Insect Biochem 18:761–770. https://doi.org/10.1016/0020-1790(88)90098-4
Birgersson G, Schlyter F, Löfqvist J, Bergström G (1984) Quantitative variation of pheromone components in the spruce bark beetle Ips typographus from different attack phases. J Chem Ecol 10:1029–1055. https://doi.org/10.1007/BF00987511
Blaženec M, Majdák A, Jakuš R (2021) Improvement of Ips typographus catches in pheromone trap barriers by altering of sex assigned pheromone blends. Folia Oecol 48(1):25–34. https://doi.org/10.2478/foecol-2021-0003
Bleiker KP, Six DL (2007) Dietary benefits of fungal associates to an eruptive herbivore: potential implications of multiple associates on host population dynamics. Environ Entomol 36:1384–1396. https://doi.org/10.1093/ee/36.6.1384
Blödner C, Majcherczyk A, Kües U, Polle A (2007) Early drought-induced changes to the needle proteome of Norway spruce. Tree Physiol 27:1423–1431. https://doi.org/10.1093/treephys/27.10.1423
Blomquist GJ, Figueroa-Teran R, Aw M, Song M, Gorzalski A, Abbott NL, Chang E, Tittiger C (2010) Pheromone production in bark beetles. Insect Biochem Mol Biol 40:699–712. https://doi.org/10.1016/j.ibmb.2010.07.013
Boone CK, Six DL, Raffa KF (2008) The enemy of my enemy is still my enemy: competitors add to predator load of a tree-killing bark beetle. Agric for Entomol 10:411–421. https://doi.org/10.1111/j.1461-9563.2008.00402.x
Boone CK, Aukema BH, Bohlmann J, Carroll AL, Raffa KF (2011) Efficacy of tree defense physiology varies with bark beetle population density: a basis for positive feedback in eruptive species. Can J for Res 41:1174–1188. https://doi.org/10.1139/x11-041
Bradshaw WE, Holzapfel CM (2006) Evolutionary response to rapid climate change. Science 312:1477–1478. https://doi.org/10.1126/science.1127000
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann for Sci 63:625–644. https://doi.org/10.1051/forest:2006042
Campbell SA, Borden JH (2006) Integration of visual and olfactory cues of hosts and nonhosts by three bark beetles (Coleoptera: Scolytidae). Ecol Entomol 31:437–449. https://doi.org/10.1111/j.1365-2311.2006.00809.x
Candotti A, De Giglio M, Dubbini M, Tomelleri E (2022) A Sentinel-2 based multi-temporal monitoring framework for wind and bark beetle detection and damage mapping. Remote Sens 14:6105. https://doi.org/10.3390/rs14236105
Carnol M, Baeten L, Branquart E, Grégoire JC, Heughebaert A, Muys B, Ponette Q, Verheyen K (2014) Ecosystem services of mixed species forest stands and monocultures: comparing practitioners’ and scientists’ perceptions with formal scientific knowledge. Forestry 87:639–653. https://doi.org/10.1093/forestry/cpu024
Caudullo G, Tinner W, de Rigo D (2016) Picea abies in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds) European atlas of forest tree species. Publication Office of the European Union, Luxembourg, pp 114–116
Chakraborty A, Ashraf MZ, Modlinger R, Synek J, Schlyter F, Roy A (2020a) Unravelling the gut bacteriome of Ips (Coleoptera: Curculionidae: Scolytinae): identifying core bacterial assemblage and their ecological relevance. Sci Rep 10:18572. https://doi.org/10.1038/s41598-020-75203-5
Chakraborty A, Modlinger R, Ashraf MZ, Synek J, Schlyter F, Roy A (2020b) Core mycobiome and their ecological relevance in the gut of five Ips bark beetles (Coleoptera: Curculionidae: Scolytinae). Front Microbiol 11:568853. https://doi.org/10.3389/fmicb.2020.568853
Chakraborty A, Purohit A, Khara A, Modlinger R, Roy A (2023) Lifestage and geographic location determine the microbial assemblage in Eurasian spruce bark beetle, Ips typographus L (Coleoptera: Curculionidae). Front for Glob Change 6:1176160. https://doi.org/10.3389/ffgc.2023.1176160
Christiaens O, Sweet J, Dzhambazova T, Urru I, Smagghe G, Kostov K, Arpaia S (2022) Implementation of RNAi-based arthropod pest control: environmental risks, potential for resistance and regulatory considerations. J Pest Sci 95:1–15. https://doi.org/10.1007/s10340-021-01439-3
Christiansen E, Waring RH, Berryman AA (1987) Resistance of conifers to bark beetle attack: searching for general relationships. For Ecol Manag 22:89–106. https://doi.org/10.1016/0378-1127(87)90098-3
Čičková H, Kozánek M, Žitňan D, Roller L (2018) Effects of γ radiation on the reproduction and enteroendocrine cells of the spruce bark beetle Ips typographus and prospects for its control. Biologia 73:67–75. https://doi.org/10.2478/s11756-018-0009-6
Cochard H, Hölttä T, Herbette S, Delzon S, Mencuccini M (2009) New insights into the mechanisms of water-stress-induced cavitation in conifers. Plant Physiol 151:949–954. https://doi.org/10.1104/pp.109.138305
Cruiziat P, Cochard H, Ameglio T (2002) Hydraulic architecture of trees: main concepts and results. Ann for Sci 59:723–752. https://doi.org/10.1051/forest:2002060
Dahlsten DL (1970) Parasites, predators, and associated organisms reared from western pine beetle infested bark samples. In: Stark RW, Dahlsten DL (eds) Studies on the population dynamics of the western pine beetle, Dendroctonus brevicomis LeConte (Coleoptera: Scolytidae). University of California, Division of Agricultural Sciences, Berkeley
Dahlsten DL, Six DL, Erbilgin N, Raffa KF, Lawson AB, Rowney DL (2003) Attraction of Ips pini (Coleoptera: Scolytidae) and its predators to various enantiomeric ratios of ipsdienol and lanierone in California: implications for the augmentation and conservation of natural enemies. Environ Entomol 32:1115–1122. https://doi.org/10.1603/0046-225X-32.5.1115
Dalponte M, Solano-Correa YT, Frizzera L, Gianelle D (2022) Mapping a European spruce bark beetle outbreak using sentinel-2 remote sensing data. Remote Sens 14:3135. https://doi.org/10.3390/rs14133135
De Groot M, Diaci J, Ogris N (2019) Forest management history is an important factor in bark beetle outbreaks: lessons for the future. For Ecol Manag 433:467–474. https://doi.org/10.1016/j.foreco.2018.11.025
De Groot M, Ogris N, Diaci J, Castagneyrol B (2023) When tree diversity does not work: the interacting effects of tree diversity, altitude and amount of spruce on European spruce bark beetle outbreaks. For Ecol Manag 537:120952. https://doi.org/10.1016/j.foreco.2023.120952
Dedrick S, Spiecker H, Orazio C, Tomé M, Martinez I (2007) Plantation or conversion–the debate. In: European forest research institute discussion paper, 13, European Forest Institute, Joensuu
Desprez-Loustau ML, Marçais B, Nageleisen LM, Piou D, Vannini A (2006) Interactive effects of drought and pathogens in forest trees. Ann for Sci 63:597–612. https://doi.org/10.1051/forest:2006040
Devos Y, Mumford JD, Bonsall MB, Camargo AM, Firbank LG, Glandorf DCM et al (2022) Potential use of gene drive modified insects against disease vectors, agricultural pests and invasive species poses new challenges for risk assessment. Crit Rev Biotechnol 42:254–270. https://doi.org/10.1080/07388551.2021.1933891
Dhandapani RK, Gurusamy D, Duan JJ, Palli SR (2020a) RNAi for management of Asian long-horned beetle, Anoplophora glabripennis: identification of target genes. J Pest Sci 93:823–832. https://doi.org/10.1007/s10340-020-01197-8
Dhandapani RK, Duan JJ, Palli SR (2020b) Orally delivered dsRNA induces knockdown of target genes and mortality in the Asian long-horned beetle, Anoplophora glabripennis. Arch Insect Biochem Physiol 104:e21679. https://doi.org/10.1002/arch.21679
Diallo S, Seck MT, Rayaissé JB, Fall AG, Bassene MD et al (2019) Chilling, irradiation and transport of male Glossina palpalis gambiensis pupae: effect on the emergence, flight ability and survival. PLoS ONE 14(5):e0216802. https://doi.org/10.1371/journal.pone.0216802
Dippel C, Heidger C, Nicolai V, Simon M (1997) The influence of four different predators on bark beetles in European forest ecosystems (Coleoptera: Scolytidae). Entomol Gener 21:161–175. https://doi.org/10.1127/entom.gen/21/1997/161
Dobor L, Hlásny T, Zimová S (2020) Contrasting vulnerability of monospecific and species-diverse forests to wind and bark beetle disturbance: the role of management. Ecol Evol 10:12233–12245. https://doi.org/10.1002/ece3.6854
Doudna JA, Charpentier E (2014) The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096. https://doi.org/10.1126/science.1258096
Drössler L, Övergaard R, Ekö PM, Gemmel P, Böhlenius H (2015) Early development of pure and mixed tree species plantations in Snogeholm, southern Sweden. Scand J for Res 30:304–316. https://doi.org/10.1080/02827581.2015.1005127
Duduman ML, Beránková K, Jakuš R, Hradecký J, Jirošová A (2022) Efficiency and sustainability of Ips duplicatus (Coleoptera: Curculionidae) pheromone dispensers with different designs. Forests 13:511. https://doi.org/10.3390/f13040511
Ebner G (2020) Significantly more damaged wood in 2019. https://www.timber-online.net/log_wood/2020/02/significantly-more-damagedwood-in-2019.html
Edmonds RL, Eglitis A (1989) The role of the Douglas-fir beetle and wood borers in the decomposition of and nutrient release from Douglas-fir logs. Can J for Res 19:853–859. https://doi.org/10.1139/x89-130
Egan JM, Jacobi WR, Negron JF, Smith SL, Cluck DR (2010) Forest thinning and subsequent bark beetle-caused mortality in Northeastern California. For Ecol Manag 260:1832-1842. https://doi.org/10.1016/j.foreco.2010.08.030
Entine J, Felipe MS, Groenewald JH, Kershen DL, Lema M, McHughen A, Nepomuceno AL, Ohsawa R, Ordonio RL, Parrott WA, Quemada H (2021) Regulatory approaches for genome edited agricultural plants in select countries and jurisdictions around the world. Transgenic Res 30:551–584. https://doi.org/10.1007/s11248-021-00257-8
Erbilgin N (2019) Phytochemicals as mediators for host range expansion of a native invasive forest insect herbivore. New Phytol 221:1268–1278. https://doi.org/10.1111/nph.15467
Erbilgin N, Raffa K (2001) Modulation of predator attraction to pheromones of two prey species by stereochemistry of plant volatiles. Oecologia 127:444–453. https://doi.org/10.1007/s004420000606
Erbilgin N, Ma C, Whitehouse C, Shan B, Najar A, Evenden M (2014) Chemical similarity between historical and novel host plants promotes range and host expansion of the mountain pine beetle in a naïve host ecosystem. New Phytol 201:940–950. https://doi.org/10.1111/nph.12573
Erbilgin N, Cale JA, Hussain A, Ishangulyyeva G, Klutsch JG, Najar A, Zhao S (2017) Weathering the storm: how lodgepole pine trees survive mountain pine beetle outbreaks. Oecologia 184:469–478. https://doi.org/10.1007/s00442-017-3865-9
Erbilgin N, Zanganeh L, Klutsch JG, Chen SH, Zhao S, Ishangulyyeva G, Burr SJ, Gaylord M, Hofstetter R, Keefover-Ring K, Raffa KF (2021) Combined drought and bark beetle attacks deplete non-structural carbohydrates and promote death of mature pine trees. Plant Cell Environ 44:3866–3881. https://doi.org/10.1111/pce.14197
European Commission (2021) New EU forest strategy for 2030. In: Communication from the commission to the European parliament, the council, the European economic and social committee and the committee of the regions, 572. European Commission, Brussels
Faccoli M, Stergulc F (2004) Ips typographus (L.) pheromone trapping in south Alps: spring catches determine damage thresholds. J Appl Entomol 128:307–311. https://doi.org/10.1111/j.1439-0418.2004.00848.x
Fares S, Mugnozza GS, Corona P, Palahí M (2015) Sustainability: five steps for managing Europe’s forests. Nature 519:407–409. https://doi.org/10.1038/519407a
Fassnacht FE, Latifi H, Ghosh A, Joshi PK, Koch B (2014) Assessing the potential of hyperspectral imagery to map bark beetle-induced tree mortality. Remote Sens Environ 140:533–548. https://doi.org/10.1016/j.rse.2013.09.014
Feicht E (2004) Parasitoids of Ips typographus (Col., Scolytidae), their frequency and composition in uncontrolled and controlled infested spruce forest in Bavaria. J Pest Sci 77:165–172. https://doi.org/10.1007/s10340-004-0047-4
Feicht E (2006) Frequency, species composition and efficiency of Ips typographus (Col., Scolytidae) parasitoids in infested spruce forests in the national park “Bavarian forest” over three consecutive years. J Pest Sci 79:35–39. https://doi.org/10.1007/s10340-005-0098-1
Fernandez KX, Pokorny S, Ishangulyeva G et al (2023) Beauveria bassiana exhibits strong virulence against Dendroctonus ponderosae in greenhouse and field experiments. Appl Microbiol Biotechnol 107:3341–3352. https://doi.org/10.1007/s00253-023-12499-z
Ferrenberg S, Kane JM, Langenhan JM (2015) To grow or defend? Pine seedlings grow less but induce more defences when a key resource is limited. Tree Physiol 35:107–111. https://doi.org/10.1093/treephys/tpv015
Fettig CJ, Hilszczański J (2015) Management strategies for bark beetles in conifer forests. In: Bark beetles. Academic Press, pp 555–584. https://doi.org/10.1016/B978-0-12-417156-5.00014-9
Fettig CJ, McKelvey SR (2010) Bark beetle responses to stand structure and prescribed fire at blacks mountain experimental forest, California, USA: 5-year data. Fire Ecol 6:26–42. https://doi.org/10.4996/fireecology.0602026
Fettig CJ, Klepzig KD, Billings RF, Munson AS, Nebeker TE, Negrón JF, Nowak JT (2007) The effectiveness of vegetation management practices for prevention and control of bark beetle infestations in coniferous forests of the western and southern United States. For Ecol Manag 238:24–53. https://doi.org/10.1016/j.foreco.2006.10.011
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811. https://doi.org/10.1038/35888
Forrester DI, Bauhus J, Cowie AL (2005) On the success and failure of mixed-species tree plantations: lessons learned from a model system of Eucalyptus globulus and Acacia mearnsii. For Ecol Manag 209:147–155. https://doi.org/10.1016/j.foreco.2005.01.012
Frewer LJ, van der Lans IA, Fischer ARH, Reinders MJ, Menozzi D, Zhang X, van den Berg I, Zimmermann KL (2013) Public perceptions of agri-food applications of genetic modification—a systematic review and meta-analysis. Trends Food Sci Technol 30:142–152. https://doi.org/10.1016/j.tifs.2013.01.003
Gao B, Yu L, Ren L, Zhan Z, Luo Y (2023) Early detection of Dendroctonus valens infestation at tree level with a Hyperspectral UAV image. Remote Sens 15:407. https://doi.org/10.3390/rs15020407
Gilles AF, Schinko JB, Averof M (2015) Efficient CRISPR-mediated gene targeting and transgene replacement in the beetle Tribolium castaneum. Development 142:2832–2839. https://doi.org/10.1242/dev.125054
Göthlin E, Schroeder LM, Lindelöw A (2000) Attacks by Ips typographus and Pityogenes chalcographus on windthrown spruces (Picea abies) during the two years following a storm felling. Scand J for Res 15:542–549. https://doi.org/10.1080/028275800750173492
Grégoire JC, Merlin J, Pasteels JM, Jaffuel R, Vouland G, Schvester D (1985) Biocontrol of Dendroctonus micans by Rhizophagus grandis Gyll. (Col., Rhizophagidae) in the Massif Central (France). Z Angew Entomol 99:182–190. https://doi.org/10.1111/j.1439-0418.1985.tb01977.x
Grégoire JC, Couillien D, Drumont A, Meyer H, Francke W (1992) Semiochemicals and the management of Rhizophagus grandis Gyll. (Col., Rhizophagidae) for the biocontrol of Dendroctonus micans Kug. (Col., Scolytidae). J Appl Entomol 114:110–112. https://doi.org/10.1111/j.1439-0418.1992.tb01103.x
Griess VC, Knoke T (2011) Growth performance, windthrow, and insects: meta-analyses of parameters influencing performance of mixed-species stands in boreal and northern temperate biomes. Can J for Res 41:1141–1159. https://doi.org/10.1139/x11-042
Guevara-Rozo S, Hussain A, Cale JA, Klutsch JG, Rajabzadeh R, Erbilgin N (2020) Nitrogen and ergosterol concentrations varied in live jack pine phloem following inoculations with fungal associates of mountain pine beetle. Front Microbiol 11:1703. https://doi.org/10.3389/fmicb.2020.01703
Gui S, Taning CNT, Wei D, Smagghe G (2020) First report on CRISPR/Cas9-targeted mutagenesis in the Colorado potato beetle, Leptinotarsa decemlineata. J Insect Physiol 121:104013. https://doi.org/10.1016/j.jinsphys.2020.104013
Gupta N, Fischer AR, Frewer LJ (2015) Ethics, risk and benefits associated with different applications of nanotechnology: a comparison of expert and consumer perceptions of drivers of societal acceptance. NanoEthics 9:93–108. https://doi.org/10.1007/s11569-015-0222-5
Gupta S, Chakraborty A, Roy A (2023) Prospects for deploying microbes against tree-killing beetles (Coleoptera) in Anthropocene. Front for Global Change 6:1182834. https://doi.org/10.3389/ffgc.2023.1182834
Hajek AE, Bauer LS (2009) Use of entomopathogens against invasive wood boring beetles in North America. In: Hajek AE, Glare TR, O’Callaghan M (eds) Use of microbes for control and eradication of invasive arthropods. Springer, Dordrecht, pp 159–179. https://doi.org/10.1016/j.jip.2012.05.008
Hanson JJ, Lorimer CG (2007) Forest structure and light regimes following moderate wind storms: Implications for multi-cohort management. Ecol Appl 17:1325–1340. https://doi.org/10.1890/06-1067.1
Harding S, Ravn H (1983) Investigation of the biology and ecology of Ips typographus (L.) in Denmark. Doctoral dissertation, PhD Thesis, University of Copenhagen
Hartley MJ (2002) Rationale and methods for conserving biodiversity in plantation forests. For Ecol Manag 155:81–95. https://doi.org/10.1016/S0378-1127(01)00549-7
Havašová M, Ferenčík J, Jakuš R (2017) Interactions between windthrow, bark beetles and forest management in the Tatra national parks. For Ecol Manag 391:349–361. https://doi.org/10.1016/j.foreco.2017.01.009
Hedgren PO, Schroeder LM (2004) Reproductive success of the spruce bark beetle Ips typographus (L.) and occurrence of associated species: a comparison between standing beetle-killed trees and cut trees. For Ecol Manag 203:241–250. https://doi.org/10.1016/j.foreco.2004.07.055
Hendrichs J, Vreysen MJB, Enkerlin WR, Cayol JP (2021) Strategic options in using sterile insects for area-wide integrated pest management. In: Sterile insect technique. CRC Press, pp 841–884
Hepper P, Wells D (2015) Olfaction in the order Carnivora: family Canidae. In: Handbook of olfaction and gustation. pp 591–604. https://doi.org/10.1002/9781118971758.ch26
Herard F, Mercadier G (1996) Natural enemies of Tomicus piniperda and Ips acuminatus (Col, scolytidae) on Pinus sylvestris near orléans, france: temporal occurrence and relative abundance, and notes on eight predatory species. Entomophaga 41:183–210. https://doi.org/10.1007/BF02764245
Herbst M, Roberts JM, Rosier PTW, Taylor ME, Gowing DJ (2007) Edge effects and forest water use: a field study in a mixed deciduous woodland. For Ecol Manag 250:176–186. https://doi.org/10.1016/j.foreco.2007.05.013
Hinze J, John R (2020) Effects of heat on the dispersal performance of Ips typographus. J Appl Entomol 144:144–151. https://doi.org/10.1111/jen.12718
Hlásny T, Zajíčková L, Turčáni M, Holuša J, Sitková Z (2011) Geographical variability of spruce bark beetle development under climate change in the Czech Republic. J for Sci 57:242–249. https://doi.org/10.17221/104/2010-jfs
Hlásny T, Trombik J, Bošeľa M, Merganič J, Marušák R, Šebeň V, Štěpánek P, Kubišta J, Trnka M (2017) Climatic drivers of forest productivity in Central Europe. Agric for Meteorol 234:258–273. https://doi.org/10.1016/j.agrformet.2016.12.024
Hlásny T, Krokene P, Liebhold A, Montagné-Huck C, Müller J, Qin H, Raffa K, Schelhaas MJ, Seidl R, Svoboda M, Viiri H (2019) Living with bark beetles: impacts, outlook and management options, From Science to Policy. https://doi.org/10.36333/fs08
Hlásny T, Zimová S, Merganičová K, Štěpánek P, Modlinger R, Turčáni M (2021) Devastating outbreak of bark beetles in the Czech Republic: drivers, impacts, and management implications. For Ecol Manag 490:119075. https://doi.org/10.1016/j.foreco.2021.119075
Hofstetter RW, Moser JC, McGuire R (2009) Observations on the mite Schizosthetus lyriformis (Acari: Parasitidae) preying on bark beetle eggs and larvae. Entomol News 120:397–400. https://doi.org/10.3157/021.120.0408
Hofstetter RW, Dinkins-Bookwalter J, Davis TS, Klepzig KD (2015) Symbiotic associations of bark beetles. In: Bark beetles: biology and ecology of native and invasive species. Elsevier Inc., Academic Press, pp 209–245. https://doi.org/10.1016/B978-0-12-417156-5.00006-X
Holuša J, Hlásny T, Modlinger R, Lukášová K, Kula E (2017) Felled trap trees as the traditional method for bark beetle control: Can the trapping performance be increased? For Ecol Manag 404:165–173. https://doi.org/10.1016/j.foreco.2017.08.019
Hood SM, Baker S, Sala A (2016) Fortifying the forest: thinning and burning increase resistance to a bark beetle outbreak and promote forest resilience. Ecol Appl 26:1984–2000. https://doi.org/10.1002/eap.1363
Hopping GR (1947) Notes on the seasonal development of Medetera Aldrichii Wheeler (Diptera: Dolichopidae) as a predator of the Douglas fir bark-beetle, Dendroctonus pseudotsugae Hopkins. Can Entomol 79:150–153. https://doi.org/10.4039/Ent79150-7
Horvath P, Barrangou R (2010) CRISPR/Cas, the immune system of bacteria and archaea. Science 327:167–170. https://doi.org/10.1126/science.1179555
Hroššo B, Mezei P, Potterf M, Majdák A, Blaženec M, Korolyova N, Jakuš R (2020) Drivers of spruce bark beetle (Ips typographus) infestations on downed trees after severe windthrow. Forests 11:1–15. https://doi.org/10.3390/f11121290
Hulcr J, Pollet M, Ubik K, Vrkoc J (2005) Exploitation of kairomones and synomones by Medetera spp. (Diptera: Dolichopodidae), predators of spruce bark beetles. Eur J Entomol 102:655–662. https://doi.org/10.14411/eje.2005.093
Huo L, Lindberg E, Bohlin J, Persson HJ (2023) Assessing the detectability of European spruce bark beetle green attack in multispectral drone images with high spatial-and temporal resolutions. Remote Sens Environ 15:113484. https://doi.org/10.1016/j.rse.2023.113484
Ikegawa Y, Ito K, Himuro C, Honma A (2021) Sterile males and females can synergistically suppress wild pests targeted by sterile insect technique. J Theor Biol 530:110878. https://doi.org/10.1016/j.jtbi.2021.110878
Ikegawa Y, Kawamura F, Sadoyama Y, Kinjo K, Haraguchi D, Honma A, Himuro C, Matsuyama T (2022) Eradication of sweet potato weevil, Cylas formicarius, from Tsuken Island, Okinawa, Japan, under transient invasion of males. J Appl Entomol 146:850–859. https://doi.org/10.1111/jen.13004
Jactel H, Brockerhoff EG (2007) Tree diversity reduces herbivory by forest insects. Ecol Lett 10:835–848. https://doi.org/10.1111/j.1461-0248.2007.01073.x
Jakuš R (1998) A method for the protection of spruce stands against Ips typographus by the use of barriers of pheromone traps in north-eastern Slovakia. Anz Schadl Pflanzenschutz Umweltschutz 71:152–158. https://doi.org/10.1007/BF02769004
Jakuš R (2001) Bark beetle (Coleoptera, Scolytidae) outbreak and system of IPM measures in an area affected by intensive forest decline connected with honey fungus (Armillaria sp.). J Pest Sci 74:46–51. https://doi.org/10.1046/j.1439-0280.2001.01008.x
Jakuš R, Blaženec M (2003) Influence of the proportion of (-) alpha-pinene in pheromone bait on Ips typographus (Col., Scolytidae) catch in pheromone trap barriers and in single traps. J Appl Entomol 127:91–95. https://doi.org/10.1046/j.1439-0418.2003.00695.x
Jakuš R, Grodzki W, Ježík M, Jachym M (2002) Definition of spatial patterns of bark beetle Ips typographus (L.) outbreak spreading in Tatra Mountains (Central Europe), using GIS. In: Proceedings: ecology, survey and management of forest insects. vol 1, pp 1–5
Jakuš R, Edwards-Jonášová M, Cudlín P, Blaženec M, Ježík M, Havlíček F, Moravec I (2011) Characteristics of Norway spruce trees (Picea abies) surviving a spruce bark beetle (Ips typographus L.) outbreak. Trees (berl. West) 25:965–973. https://doi.org/10.1007/s00468-011-0571-9
Jakuš R, Modlinger R, Kašpar J, Majdák A, Blaženec M, Korolyova N, Jirošová A, Schlyter F (2022) Testing the efficiency of the push-and-pull strategy during severe Ips typographus outbreak and extreme drought in Norway spruce stands. Forests 13:2175. https://doi.org/10.3390/f13122175
Jakuš R, Trubin A, Singh VV, Zabihi K, Jirošová A, Modlinger R, Majdák A et al (2024) Spruce protection against Ips typographus with anti-attractant blend of tree-based semiochemicals: From small experimental plots to stand scales. Forests 15:356. https://doi.org/10.3390/f15020356
Jamali S, Olsson PO, Ghorbanian A, Müller M (2023) Examining the potential for early detection of spruce bark beetle attacks using multi-temporal Sentinel-2 and harvester data. ISPRS J Photogramm Remote Sens 1:352–366. https://doi.org/10.1016/j.isprsjprs.2023.10.013
Jiang F, Doudna JA (2017) CRISPR–Cas9 structures and mechanisms. Ann Rev Biophys 46:505–529. https://doi.org/10.1146/annurev-biophys-062215-010822
Jirošová A, Kalinová B, Modlinger R, Jakuš R, Unelius CR, Blaženec M, Schlyter F (2022) Anti-attractant activity of (+)-trans-4-thujanol for Eurasian spruce bark beetle Ips typographus: novel potency for females. Pest Manag Sci 78:1992–1999. https://doi.org/10.1002/ps.6819
Joga MR, Zotti MJ, Smagghe G, Christiaens O (2016) RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: what we know so far. Front Physiol 7:553. https://doi.org/10.3389/fphys.2016.00553
Joga MR, Mogilicherla K, Smagghe G, Roy A (2021) RNA interference-based forest protection products (FPPs) against wood-boring coleopterans: Hope or hype? Front Plant Sci 12:733608. https://doi.org/10.3389/fpls.2021.733608
Johansson AM, Birgersson G, Schlyter F (2019) Using synthetic semiochemicals to train canines to detect bark beetle–infested trees. Ann Forest Sci 76:262386. https://doi.org/10.1007/s13595-019-0841-z
Jones KL, Shegelski VA, Marculis NG, Wijerathna AN, Evenden ML (2019) Factors influencing dispersal by flight in bark beetles (Coleoptera: Curculionidae: Scolytinae): from genes to landscapes. Can J for Res 49:1024–1041. https://doi.org/10.1139/cjfr-2018-0304
Kärvemo S, Van Boeckel TP, Gilbert M, Grégoire JC, Schroeder M (2014) Large-scale risk mapping of an eruptive bark beetle—importance of forest susceptibility and beetle pressure. For Ecol Manag 318:158–166. https://doi.org/10.1016/j.foreco.2014.01.025
Kautz M, Dworschak K, Gruppe A, Schopf R (2011) Quantifying spatio-temporal dispersion of bark beetle infestations in epidemic and non-epidemic conditions. For Ecol Manag 262:598–608. https://doi.org/10.1016/j.foreco.2011.04.023
Kautz M, Schopf R, Ohser J (2013) The “sun-effect”: microclimatic alterations predispose forest edges to bark beetle infestations. Eur J for Res 132:453–465. https://doi.org/10.1007/s10342-013-0685-2
Kautz M, Schopf R, Imron MA (2014) Individual traits as drivers of spatial dispersal and infestation patterns in a host–bark beetle system. Ecol Model 273:264–276. https://doi.org/10.1016/j.ecolmodel.2013.11.022
Kautz M, Feurer J, Adler P (2024) Early detection of bark beetle (Ips typographus) infestations by remote sensing—a critical review of recent research. For Ecol Manag 556:121595. https://doi.org/10.1016/j.foreco.2023.121595
Keeling CI, Tittiger C, MacLean M, Blomquist GJ (2021) Pheromone production in bark beetles. In: Insect pheromone biochemistry and molecular biology. Academic Press, pp 123–162. https://doi.org/10.1016/B978-0-12-819628-1.00004-3
Kenis M, Wermelinger B, Grégoire JC (2007) Research on parasitoids and predators of Scolytidae—a review. In: Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-2241-8_11
Kenis M, Hurley BP, Hajek AE, Cock MJ (2017) Classical biological control of insect pests of trees: facts and figures. Biol Invasions 19:3401–3417. https://doi.org/10.1007/s10530-017-1414-4
Klapwijk MJ, Björkman C (2018) Mixed forests to mitigate risk of insect outbreaks. Scand J for Res 33:772–780. https://doi.org/10.1080/02827581.2018.1502805
Klapwijk MJ, Bylund H, Schroeder M, Björkman C (2016) Forest management and natural biocontrol of insect pests. Forestry 89:253–262. https://doi.org/10.1093/forestry/cpw019
Klassen W, Vreysen MJB (2021) Area-wide integrated pest management and the sterile insect technique. In: Sterile insect technique. CRC Press, pp 75–112
Klutsch JG, Cale JA, Whitehouse C, Kanekar SS, Erbilgin N (2017) Trap trees: an effective method for monitoring mountain pine beetle activities in novel habitats. Can J for Res 47:1432–1437. https://doi.org/10.1139/cjfr-2017-0189
Knapp EE, Bernal AA, Kane JM, Fettig CJ, North MP (2021) Variable thinning and prescribed fire influence tree mortality and growth during and after a severe drought. For Ecol Manag 479:118595. https://doi.org/10.1016/j.foreco.2020.118595
Kohnle U, Vité JP (1984) Bark beetle predators: Strategies in the olfactory perception of prey species by clerid and trogositid beetles. Z Für Angew 98:504–508. https://doi.org/10.1111/j.1439-0418.1984.tb02741.x
Korecký J, Čepl J, Korolyova N, Stejskal J, Turčáni J, Jakuš R (2023) Resilience to bark beetle infestation in Norway spruce: Population structure analysis and comparative genomic assessment of surviving (LTS) and randomly selected reference trees. Forests 14:2074. https://doi.org/10.3390/f14102074
Korolyova N, Buechling A, Lieutier F, Yart A, Cudlín P, Turčáni M, Jakuš R (2022) Primary and secondary host selection by Ips typographus depends on Norway spruce crown characteristics and phenolic-based defenses. Plant Sci 321:111319. https://doi.org/10.1016/j.plantsci.2022.111319
Koštál V, Doležal P, Rozsypal J, Moravcová M, Zahradníčková H, Šimek P (2011) Physiological and biochemical analysis of overwintering and cold tolerance in two Central European populations of the spruce bark beetle, Ips typographus. J Insect Physiol 57:1136–1146. https://doi.org/10.1016/j.jinsphys.2011.03.011
Kreutz J, Zimmermann G, Vaupel O (2004) Horizontal transmission of the entomopathogenic fungus Beauveria bassiana among the spruce bark beetle, Ips typographus (Col., Scolytidae) in the laboratory and under field conditions. Biocontrol Sci Technol 14:837–848. https://doi.org/10.1080/788222844
Krokene P (2015) Conifer defense and resistance to bark beetles. In: Bark beetles: biology and ecology of native and invasive species. Academic Press, pp 177–207. https://doi.org/10.1016/B978-0-12-417156-5.00005-8
Kyre BR, Rieske LK (2022) Using RNAi to silence heat shock protein has congeneric effects in North America’s Dendroctonus bark beetles. For Ecol Manag 520:120367. https://doi.org/10.1016/j.foreco.2022.120367
Kyre BR, Rodrigues TB, Rieske LK (2019) RNA interference and validation of reference genes for gene expression analyses using qPCR in southern pine beetle, Dendroctonus frontalis. Sci Rep 9:5640. https://doi.org/10.1038/s41598-019-42072-6
Kyre BR, Bentz BJ, Rieske LK (2020) Susceptibility of mountain pine beetle (Dendroctonus ponderosae Hopkins) to gene silencing through RNAi provides potential as a novel management tool. For Ecol Manag 473:118322. https://doi.org/10.1016/j.foreco.2020.118322
Kyre BR, Dupuis JR, Zúñiga G, Rieske LK (2024) Variation in RNA interference sensitivity in the southern pine beetle, Dendroctonus frontalis (Coleoptera: Curculionidae). Biol J Linn Soc. https://doi.org/10.1093/biolinnean/blad126
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41. https://doi.org/10.1016/j.jip.2015.07.009
Lance DR, McInnis DO (2005) Biological basis of the sterile insect technique. Sterile Insect Tech Princ Pract Area-Wide Integr Pest Manag. https://doi.org/10.1007/1-4020-4051-2_3
Lausch A, Fahse L, Heurich M (2011) Factors affecting the spatio-temporal dispersion of Ips typographus (L.) in Bavarian Forest National Park: a long-term quantitative landscape-level analysis. For Ecol Manag 261:233–245. https://doi.org/10.1016/j.foreco.2010.10.012
Lawson SA, Morgan FD (1992) Rearing of two predators, Thanasimus dubius and Temnochila virescens, for the biological control of Ips grandicollis in Australia. Entomol Exp Appl 65:225–233. https://doi.org/10.1111/j.1570-7458.1992.tb00675.x
Lawson SA, Furuta K, Katagiri K (1996) The effect of host tree on the natural enemy complex of Ips typographus japonicus Niijima (Col., Scolytidae) in Hokkaido, Japan. J Appl Entomol 120:77–86. https://doi.org/10.1111/j.1439-0418.1996.tb01570.x
Lawson SA, Furuta K, Katagiri K (1997) Effect of natural enemy exclusion on mortality of Ips typographus japonicus Niijima (Col., Scolytidae) in Hokkaido, Japan. J Appl Entomol 121:89–98. https://doi.org/10.1111/j.1439-0418.1997.tb01376.x
Le Provost G, Domergue F, Lalanne C, Ramos Campos P, Grosbois A, Bert D, Meredieu C, Danjon F, Plomion C, Gion JM (2013) Soil water stress affects both cuticular wax content and cuticle-related gene expression in young saplings of maritime pine (Pinus pinaster Ait). BMC Plant Biol 13:1–12. https://doi.org/10.1186/1471-2229-13-95
Lefcheck J, Byrnes J, Isbell F, Gamfeldt L, Griffin JN, Eisenhauer N, Hensel M, Hector A, Cardinale BJ, Duffy E (2015) Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat Commun 6:6936. https://doi.org/10.1038/ncomms7936
Li S, Kim DS, Zhang J (2023) Plastid-mediated RNA interference: a potential strategy for efficient pest control. Plant Cell Environ. https://doi.org/10.1111/pce.14652
Lieutier F, Yart A, Salle A (2009) Stimulation of tree defenses by ophiostomatoid fungi can explain attack success of bark beetles on conifers. Ann for Sci 66:801. https://doi.org/10.1051/forest/2009066
Liu CLC, Kuchma O, Krutovsky KV (2018) Mixed-species versus monocultures in plantation forestry: development, benefits, ecosystem services and perspectives for the future. Glob Ecol Conserv 15:e00419. https://doi.org/10.1016/j.gecco.2018.e00419
Lohbeck M, Bongers F, Martinez-Ramos M, Poorter L (2016) The importance of biodiversity and dominance for multiple ecosystem functions in a human-modified tropical landscape. Ecology 97:2772–2779. https://doi.org/10.1002/ecy.1499
Lombardero MJ, Ayres MP, Ayres BD, Reeve JD (2000) Cold tolerance of four species of bark beetle (Coleoptera: Scolytidae) in North America. Environ Entomol 29:421–432. https://doi.org/10.1603/0046-225X-29.3.421
Long JA, Lawrence RL (2016) Mapping percent tree mortality due to mountain pine beetle damage. For Sci 62:392–402. https://doi.org/10.5849/forsci.15-046
Lubojacký J, Holuša J (2014) Attraction of Ips typographus (Coleoptera: Curculionidae) beetles by lure-baited insecticide-treated tripod trap logs and trap trees. Int J Pest Manag 60:153–159. https://doi.org/10.1080/09670874.2014.944610
Mann AJ, Davis TS (2021) Entomopathogenic fungi to control bark beetles: a review of ecological recommendations. Pest Manag Sci 77:3841–3846. https://doi.org/10.1002/ps.6364
Marešová J, Majdák A, Jakuš R, Hradecký J, Kalinová B, Blaženec M (2020) The short-term effect of sudden gap creation on tree temperature and volatile composition profiles in a Norway spruce stand. Trees 34:1397–1409. https://doi.org/10.1007/s00468-020-02010-w
Marini L, Ayres MP, Battisti A, Faccoli M (2012) Climate affects severity and altitudinal distribution of outbreaks in an eruptive bark beetle. Clim Change 115:327–341. https://doi.org/10.1007/s10584-012-0463-z
Marini L, Økland B, Jönsson AM, Bentz B, Carroll A, Forster B, Grégoire JC, Hurling R, Nageleisen LM, Netherer S, Ravn HP, Weed A, Schroeder M (2017) Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 40:1426–1435. https://doi.org/10.1111/ecog.02769
Martín A, Etxebeste I, Pérez G, Álvarez G, Sánchez E, Pajares J (2013) Modified pheromone traps help reduce bycatch of bark-beetle natural enemies. Agric for Entomol 15:86–97. https://doi.org/10.1111/j.1461-9563.2012.00594.x
Marvasti-Zadeh SM, Goodsman D, Ray N, Erbilgin N (2024) Early detection of bark beetle attack using remote sensing and machine learning: a review. ACM Comput Surv 56:4. https://doi.org/10.1145/3625387
Mat Jalaluddin NS, Othman RY, Harikrishna JA (2019) Global trends in research and commercialization of exogenous and endogenous RNAi technologies for crops. Crit Rev Biotechnol 39:67–78. https://doi.org/10.1080/07388551.2018.1496064
Matthews B, Netherer S, Katzensteiner K, Pennerstorfer J, Blackwell E, Henschke P et al (2018) Transpiration deficits increase host susceptibility to bark beetle attack: experimental observations and practical outcomes for Ips typographus hazard assessment. Agri for Meteorol 263:69–89. https://doi.org/10.1016/j.agrformet.2018.08.004
Máximo WPF, Howell JL, Mogilicherla K, Basij M, Chereddy SCRR, Palli SR (2020) Inhibitor of apoptosis is an effective target gene for RNAi-mediated control of Colorado potato beetle, Leptinotarsa decemlineata. Arch Insect Biochem Physiol 104:e21685. https://doi.org/10.1002/arch.21685
Mazón MM, Klanderud K, Finegan B, Veintimilla D, Bermeo D, Murrieta E, Delgado D, Sheil D (2020) How forest structure varies with elevation in old growth and secondary forest in Costa Rica. For Ecol Manag 469:118191. https://doi.org/10.1016/j.foreco.2020.118191
Meddens AJH, Hicke JA (2014) Spatial and temporal patterns of landsat-based detection of tree mortality caused by a mountain pine beetle outbreak in Colorado, USA. For Ecol Manag 322:78–88. https://doi.org/10.1016/j.foreco.2014.02.037
Mezei P, Grodzki W, Blaženec M, Škvarenina J, Brandýsová V, Jakuš R (2014a) Host and site factors affecting tree mortality caused by the spruce bark beetle (Ips typographus) in mountainous conditions For. Ecol Manag 331:196–207. https://doi.org/10.1016/j.foreco.2014.07.031
Mezei P, Grodzki W, Blaženec M, Jakuš R (2014b) Factors influencing the wind-bark beetles’ disturbance system in the course of an Ips typographus outbreak in the Tatra Mountains. For Ecol Manag 312:67–77. https://doi.org/10.1016/j.foreco.2013.10.020
Mezei P, Jakuš R, Pennerstorfer J, Havašová M, Škvarenina J, Ferenčík J, Slivinský J, Bičárová S, Bilčík D, Blaženec M, Netherer S (2017) Storms, temperature maxima and the Eurasian spruce bark beetle Ips typographus—an infernal trio in Norway spruce forests of the Central European High Tatra Mountains. Agric for Meteorol 242:85–95. https://doi.org/10.1016/j.agrformet.2017.04.004
Mezzetti B, Smagghe G, Arpaia S, Christiaens O, Dietz-Pfeilstetter A, Jones H, Kostov K et al (2020) RNAi: What is its position in agriculture? J Pest Sci 93:1125–1130. https://doi.org/10.1007/s10340-020-01238-2
Mezzetti B, Arpaia S, Baraldi E, Dietz-Pfeilstetter A, Smagghe G, Ventura V, Sweet JB (2022) Advances and challenges of RNAi based technologies for plants—volume 2. Front Plant Sci 13:930851. https://doi.org/10.3389/fpls.2022.930851
Mills NJ (1985) Some observations on the role of predation in the natural regulation of Ips typographus populations. Z Für Angew 99:209–215. https://doi.org/10.1111/j.1439-0418.1985.tb01980.x
Mogilicherla K, Roy A (2023a) Epigenetic regulations as drivers of insecticide resistance and resilience to climate change in arthropod pests. Front Genet 13:1044980. https://doi.org/10.3389/fgene.2022.1044980
Mogilicherla K, Roy A (2023b) RNAi-chitosan biopesticides for managing forest insect pests: an outlook. Front for Glob Change 6:1219685. https://doi.org/10.3389/ffgc.2023.1219685
Mosconi F, Campanaro A, Carpaneto GM, Chiari S, Hardersen S, Mancini E, Sabatelli S, Zauli A, Mason F, Audisio P (2017) Training of a dog for the monitoring of Osmoderma eremita. Nat Conserv 20:237–264. https://doi.org/10.3897/natureconservation.20.12688
Moser JC (1975) Mite Predators of the Southern Pine Beetle. Ann Entomol Soc Am 68:1113–1116. https://doi.org/10.1093/aesa/68.6.1113
Moser JC, Roton LM (1971) Mites associated with southern pine bark beetles in Allen Parish, Louisiana. Can Entomol 103:1775–1798. https://doi.org/10.4039/Ent1031775-12
Moser JC, Kiełczewski B, Wiśniewski J, Bałazy S (1978) Evaluating Pyemotes dryas (Vitzthum 1923) (Acari: Pyemotidae) as a parasite of the southern pine beetle. Int J Acarol 4:67–70. https://doi.org/10.1080/01647957808684026
Moser JC, Eidmann HH, Regnander JR (1989) The mites associated with Ips typographus in Sweden. Ann Entomol Fenn 55:23–27
Moser JC, Konrad H, Kirisits T, Carta LK (2005) Phoretic mites and nematode associates of Scolytus multistriatus and Scolytus pygmaeus (Coleoptera: Scolytidae) in Austria. Agric for Entomol 7:169–177. https://doi.org/10.1111/j.1461-9555.2005.00261.x
Muratoğlu H, Sezem K, Demirbağ Z (2011) Determination and pathogenicity of the bacterial flora associated with the spruce bark beetle, Ips typographus (L.) (Coleoptera: Curculionidae: Scolytinae). Turk J Biol 35:9–20. https://doi.org/10.3906/biy-0902-12
Nardi D, Bozzini A, Morgante G, Gaccione A, Finozzi V, Battisti A (2023) Participatory ground data are complementary to satellite bark beetle detection. Ann for Sci 80:46. https://doi.org/10.1186/s13595-023-01216-5
Naseer A, Mogilicherla K, Sellamuthu G, Roy A (2023) Age matters: life-stage, tissue, and sex-specific gene expression dynamics in Ips typographus (Coleoptera: Curculionidae: Scolytinae). Front for Glob Change. https://doi.org/10.3389/ffgc.2023.1124754
Nealis VG, Peter B (2008) Risk assessment of the threat of mountain pine beetle to Canada’s boreal and eastern pine resources. https://d1ied5g1xfgpx8.cloudfront.net/pdfs/28891.pdf
Nelson TA, Boots B, Wulder MA, Carroll AL (2007) Environmental characteristics of mountain pine beetle infestation hot spots. J Ecosyst Manag 8:91–108. https://doi.org/10.22230/jem.2007v8n1a367
Netherer S, Matthews B, Katzensteiner K, Blackwell E, Henschke P, Hietz P, Pennerstorfer J, Rosner S, Kikuta S, Schume H, Schopf A (2014) Do water-limiting conditions predispose Norway spruce to bark beetle attack? New Phytol 205:1128–1141. https://doi.org/10.1111/nph.13166
Netherer S, Panassiti B, Pennerstorfer J, Matthews B (2019) Acute drought is an important driver of bark beetle infestation in Austrian Norway spruce stands. Front for Glob Change 2:39. https://doi.org/10.3389/ffgc.2019.00039
Netherer S, Lehmanski L, Bachlehner A, Rosner S, Savi T, Schmidt A, Huang J, Paiva MR, Mateus E, Hartmann H, Gershenzon J (2024) Drought increases Norway spruce susceptibility to the Eurasian spruce bark beetle and its associated fungi. New Phytol. https://doi.org/10.1111/nph.19635
Nicolai V (1995) The impact of Medetera dendrobaena Kowarz (Dipt., Dolichopodidae) on bark beetles. J Appl Entomol 119:161–166. https://doi.org/10.1111/j.1439-0418.1995.tb01264.x
Niemann KO, Quinn G, Stephen R, Visintini F, Parton D (2015) Hyperspectral remote sensing of mountain pine beetle with an emphasis on previsual assessment. Can J Remote Sens 41:191–202. https://doi.org/10.1080/07038992.2015.1065707
Niemeyer HA (1997) Integrated bark beetle control: experiences and problems in Northern Germany. In: Proceedings: integrating cultural tactics into the management of bark beetle and reforestation pests; Vallombrosa, U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, Radnor, PA, USDA Forest Service General Technical Report NE. 236: 80–86
Ogaugwu CE, Schetelig MF, Wimmer EA (2013) Transgenic sexing system for Ceratitis capitata (Diptera: Tephritidae) based on female-specific embryonic lethality. Insect Biochem Mol Biol 43:1–8. https://doi.org/10.1016/j.ibmb.2012.10.010
Öhrn P (2012) Seasonal flight patterns of the spruce bark beetle (Ips typographus) in Sweden—phenology, Voltinism and Development. Licentiate Thesis. Department of Ecology, Swedish University of Agricultural Sciences, Sweden. https://res.slu.se/id/publ/79084
Öhrn P, Långström B, Lindelöw Å, Björklund N (2014) Seasonal flight patterns of Ips typographus in southern Sweden and thermal sums required for emergence. Agric for Entomol 16:147–157. https://doi.org/10.1111/afe.12044
Økland B, Berryman A (2004) Resource dynamic plays a key role in regional fluctuations of the spruce bark beetles Ips typographus. Agric for Entomol 6:141–146. https://doi.org/10.1111/j.1461-9555.2004.00214.x
Økland B, Bjørnstad ON (2006) A resource-depletion model of forest insect outbreaks. Ecology 87:283–290. https://doi.org/10.1890/05-0135
Økland B, Nikolov C, Krokene P, Vakula J (2016) Transition from windfall- to patch-driven outbreak dynamics of the spruce bark beetle Ips typographus. For Ecol Manag 363:63–73. https://doi.org/10.1016/j.foreco.2015.12.007
Otvos IS (1965) Studies on avian predators of Dendroctonus brevicomis LeConte (Coleoptera: Scolytidae) with special reference to Picidae. Can Entomol 97:1184–1199. https://doi.org/10.4039/Ent971184-11
Özçelik MS, Tomášková I, Surový P, Modlinger R (2022) Effect of forest edge cutting on transpiration rate in Picea abies (L) H. Karst. Forests 13:1238. https://doi.org/10.3390/f13081238
Pallis S, Alyokhin A, Manley B, Rodrigues T, Barnes E, Narva K (2023) Effects of low doses of a novel dsRNA-based biopesticide (Calantha) on the Colorado potato beetle. J Econ Entomol 116:456–461. https://doi.org/10.1093/jee/toad034
Pawson SM, Brin A, Brockerhoff EG, Lamb D, Payn TW, Paquette A, Parrotta JA (2013) Plantation forests, climate change and biodiversity. Biodivers Conserv 22:1203–1227. https://doi.org/10.1007/s10531-013-0458-8
Perkin LC, Adrianos SL, Oppert B (2016) Gene disruption technologies have the potential to transform stored product insect pest control. InSects 7:46. https://doi.org/10.3390/insects7030046
Podevin N, Davies HV, Hartung F, Nogué F, Casacuberta JM (2013) Site-directed nucleases: a paradigm shift in predictable, knowledge-based plant breeding. Trends Biotechnol 31:375–383. https://doi.org/10.1016/j.tibtech.2013.03.004
Popa V, Deziel E, Lavallée R, Bauce E, Guertin C (2012) The complex symbiotic relationships of bark beetles with microorganisms: a potential practical approach for biological control in forestry. Pest Manag Sci 68:963–975. https://doi.org/10.1002/ps.3307
Potterf M, Nikolov C, Kočická E, Ferenčík J, Mezei P, Jakuš R (2019) Landscape-level spread of beetle infestations from windthrown- and beetle-killed trees in the non-intervention zone of the Tatra National Park, Slovakia (Central Europe). For Ecol Manag 432:489–500. https://doi.org/10.1016/j.foreco.2018.09.050
Powell JA, Logan JA (2005) Insect seasonality: Circle map analysis of temperature-driven life cycles. Theor Popul Biol 67:161–179. https://doi.org/10.1016/j.tpb.2004.10.001
Powell D, Groβe-Wilde E, Krokene P, Roy A, Chakraborty A, Löfstedt C, Vogel H, Andersson MN, Schlyter F (2021) A highly-contiguous genome assembly of the Eurasian spruce bark beetle, Ips typographus, provides insight into a major forest pest. Commun Biol 4:1059. https://doi.org/10.1038/s42003-021-02602-3
Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58:501–517. https://doi.org/10.1641/B580607
Raffa KF, Grégoire JC, Lindgren BS (2015) Natural history and ecology of bark beetles. In: Bark beetles: biology and ecology of native and invasive species. Elsevier Inc., pp 1–40. https://doi.org/10.1016/B978-0-12-417156-5.00001-0
Ramakrishnan R, Hradecký J, Roy A, Kalinová B, Mendezes RC, Synek J, Bláha J, Svatoš A, Jirošová A (2022) Metabolomics and transcriptomics of pheromone biosynthesis in an aggressive forest pest Ips typographus. Insect Biochem Mol Biol 140:103680. https://doi.org/10.1016/j.ibmb.2021.103680
Ranade SS, Delhomme N, García-Gil MR (2019) Transcriptome analysis of shade avoidance and shade tolerance in conifers. Planta 250:299–318. https://doi.org/10.1007/s00425-019-03160-z
Ranade SS, Seipel G, Gorzsás A, García-Gil MR (2022) Enhanced lignin synthesis and ecotypic variation in defense-related gene expression in response to shade in Norway spruce. Plant Cell Environ 45:2671–2681. https://doi.org/10.1111/pce.14387
Rank AP, Koch A (2021) Lab-to-field transition of RNA spray applications—How far are we? Front Plant Sci 12:755203. https://doi.org/10.3389/fpls.2021.755203
Raty L, Drumont A, De Windt N, Grégoire JC (1995) Mass trapping of the spruce bark beetle Ips typographus L.: Traps or trap trees? For Ecol Manage 78:191–205. https://doi.org/10.1016/0378-1127(95)03582-1
Reeve J (1997) Predation and bark beetle dynamics. Oecologia 112:48–54. https://doi.org/10.1007/s004420050282
Rennenberg H, Loreto F, Polle A, Brilli F, Fares S, Beniwal RS, Gessler A (2006) Physiological responses of forest trees to heat and drought. Plant Biol 8:556–571. https://doi.org/10.1055/s-2006-924084
Richter H, Randau L, Plagens A (2013) Exploiting CRISPR/Cas: interference mechanisms and applications. Int J Mol Sci 14:14518–14531. https://doi.org/10.3390/ijms140714518
Rissanen K, Hölttä T, Vanhatalo A, Aalto J, Nikinmaa E, Rita H, Bäck J (2016) Diurnal patterns in Scots pine stem oleoresin pressure in a boreal forest. Plant Cell Environ 39:527–538. https://doi.org/10.1111/pce.12637
Roberts A, Dragicevic S, Northrup J, Wolf S, Li Y, Coburn C (2003) Mountain pine beetle detection and monitoring: remote sensing evaluations.-BC Forest Innovation Investment Operational Research Report with Reference to Recipient Agreement R2003–0205
Robinson AS (2021) Genetic basis of the sterile insect technique. In: Sterile insect technique. CRC Press, pp 143–162
Rodrigues TB, Rieske LK, Duan J, Mogilicherla K, Palli SR (2017a) Development of RNAi method for screening candidate genes to control emerald ash borer, Agrilus planipennis. Sci Rep 7:7379. https://doi.org/10.1038/s41598-017-07605-x
Rodrigues TB, Dhandapani R, Duan J, Palli SR (2017b) RNA interference in the Asian longhorned beetle: Identification of key RNAi genes and reference genes for RT-qPCR. Sci Rep 7:8913. https://doi.org/10.1038/s41598-017-08813-1
Rodrigues TB, Duan JJ, Palli SR, Rieske LK (2018) Identification of highly effective target genes for RNAi-mediated control of emerald ash borer, Agrilus planipennis. Sci Rep 8:5020. https://doi.org/10.1038/s41598-018-23216-6
Rodriguez R, Redman R (2008) More than 400 million years of evolution and some plants still can’t make it on their own: plant stress tolerance via fungal symbiosis. J Exp Bot 59:1109–1114. https://doi.org/10.1093/jxb/erm342
Rosana AR, Pokorny S, Klutsch JG et al (2021) Selection of entomopathogenic fungus Beauveria bassiana (Deuteromycotina: Hyphomycetes) for the biocontrol of Dendroctonus ponderosae (Coleoptera: Curculionidae, Scolytinae) in Western Canada. Appl Microbiol Biotechnol 105:2541–2557. https://doi.org/10.1007/s00253-021-11172-7
Rothe M, Vogel M, Roloff A (2002) Characterisation of the water relations of spruce (Picea abies (L.) Karst) during prolonged artificial drought stress. Allg Forst Jagdztg 173:29–36. https://doi.org/10.5555/20023059225
Ruel JC, Wermelinger B, Gauthier S, Burton PJ, Waldron K, Shorohova E (2023) Selected examples of interactions between natural disturbances. In: Girona MM, Morin H, Gauthier S, Bergeron Y (eds) Boreal forests in the face of climate change: advances in global change research, vol 74. Springer. https://doi.org/10.1007/978-3-031-15988-6_4
Safonova A, Hamad Y, Alekhina A, Kaplun D (2022) Detection of Norway spruce trees (Picea abies) infested by bark beetle in UAV images using YOLOs architectures. IEEE Access 10:10384–10392. https://doi.org/10.1109/ACCESS.2022.3144433
Safranyik L, Carroll AL (2007) The biology and epidemiology of the mountain pine beetle in lodgepole pine forests. The mountain pine beetle: a synthesis of biology, management and impacts on lodgepole pine, 3–66. https://www.cabdirect.org/cabdirect/abstract/20073248170
Sarikaya O, Avci M (2009) Predators of Scolytinae (Coleoptera: Curculionidae) species of the coniferous forests in the Western Mediterranean Region, Turkey. Turkiye Entomoloji Dergisi—Turkish J Entomol 33:253–264
Schiebe C, Blaženec M, Jakuš R, Unelius CR, Schlyter F (2011) Semiochemical diversity diverts bark beetle attacks from Norway spruce edges. J Appl Entomol 135:726–737. https://doi.org/10.1111/j.1439-0418.2011.01624.x
Schiebe C, Unelius CR, Ganji S, Binyameen M, Birgersson G, Schlyter F (2019) Styrene, (+)-trans-(1R,4S,5S)-4-thujanol and oxygenated monoterpenes related to host stress elicit strong electrophysiological responses in the bark beetle Ips typographus. J Chem Ecol 45:474–489. https://doi.org/10.1007/s10886-019-01070-8
Schlyter F, Byers JA, Löfqvist J (1987) Attraction to pheromone sources of different quantity, quality, and spacing: density-regulation mechanisms in bark beetle Ips typographus. J Chem Ecol 13:1503–1523. https://doi.org/10.1007/BF01012294
Schume H, Jost G, Hager H (2004) Soil water depletion and recharge patterns in mixed and pure forest stands of European beech and Norway spruce. J Hydrol 289:258–274. https://doi.org/10.1016/j.jhydrol.2003.11.036
Seidel H, Schunk C, Matiu M, Menzel A (2016) Diverging drought resistance of scots pine provenances revealed by infrared thermography. Front Plant Sci 7:1247. https://doi.org/10.3389/fpls.2016.01247
Seidl R, Schelhaas MJ, Lexer MJ (2011) Unraveling the drivers of intensifying forest disturbance regimes in Europe. Glob Chang Biol 17:2842–2852. https://doi.org/10.1111/j.1365-2486.2011.02452.x
Seidl R, Schelhaas MJ, Rammer W, Verkerk PJ (2014) Increasing forest disturbances in Europe and their impact on carbon storage. Nat Clim Chang 4:806–810. https://doi.org/10.1038/nclimate2318
Sellamuthu G, Naseer A, Hradecký J, Chakraborty A, Synek J, Modlinger R, Roy A (2023) Gene expression plasticity facilitates different host feeding in Ips sexdentatus (Coleoptera: Curculionidae: Scolytinae). Insect Biochem Mol Biol. https://doi.org/10.1016/j.ibmb.2023.10406
Senf C, Pflugmacher D, Wulder MA, Hostert P (2015) Characterizing spectral–temporal patterns of defoliator and bark beetle disturbances using Landsat time series. Remote Sens Environ 170:166–177. https://doi.org/10.1016/j.rse.2015.09.019
Senf C, Pflugmacher D, Hostert P, Seidl R (2017) Using Landsat time series for characterizing forest disturbance dynamics in the coupled human and natural systems of Central Europe. ISPRS J Photogramm Remote Sens 130:453–463. https://doi.org/10.1016/j.isprsjprs.2017.07.004
Shew AM, Danforth DM, Nalley LL, Nayga RM Jr, Tsiboe F, Dixon BL (2017) New innovations in agricultural biotech: consumer acceptance of topical RNAi in rice production. Food Control 81:189–195. https://doi.org/10.1016/j.foodcont.2017.05.047
Simard M, Powell EN, Raffa KF, Turner MG (2012) What explains landscape patterns of tree mortality caused by bark beetle outbreaks in Greater Yellowstone?: Landscape patterns of bark beetle outbreaks. Glob Ecol Biogeogr 21:556–567. https://doi.org/10.1111/j.1466-8238.2011.00710.x
Singh S, Rahangdale S, Pandita S, Saxena G, Upadhyay SK, Mishra G, Verma PC (2022) CRISPR/Cas9 for insect pests management: a comprehensive review of advances and applications. Agriculture 12:1896. https://doi.org/10.3390/agriculture12111896
Singh VV, Zabihi K, Trubin A, Jakuš R, Korolyova N, Cudlín P, Blaženec M (2023) Effect of diurnal solar radiation regime and tree density on sap flow of Norway spruce (Picea abies [L.] Karst.) in fragmented stands. https://doi.org/10.21203/rs.3.rs-3262723/v1
Singh VV, Naseer A, Sellamuthu G, Jakuš R (2024) An optimized and cost-effective RNA extraction method for secondary metabolite-enriched tissues of norway spruce (Picea abies). Plants 13:389. https://doi.org/10.3390/plants13030389
Sousa M, Birgersson G, Karlsson Green K, Pollet M, Becher PG (2023) Odors attracting the long-legged predator Medetera signaticornis Loew to Ips typographus L infested Norway spruce trees. J Chem Ecol. https://doi.org/10.1007/s10886-023-01405-6
Spiecker H (2000) Growth of Norway spruce (Picea abies L. karst.) under changing environmental conditions in Europe. In: Klimo E, Hager H, Kulhavy J (eds) Spruce monocultures in Central Europe—problems and prospects. pp 11–26. https://doi.org/10.5555/20003015533
Sproull GJ, Adamus M, Bukowski M, Krzyżanowski T, Szewczyk J, Statwick J, Szwagrzyk J (2015) Tree and stand-level patterns and predictors of Norway spruce mortality caused by bark beetle infestation in the Tatra Mountains. For Ecol Manag 354:261–271. https://doi.org/10.1016/j.foreco.2015.06.006
Stadelmann G, Bugmann H, Meier F, Wermelinger B, Bigler C (2013) Effects of salvage logging and sanitation felling on bark beetle (Ips typographus L.) infestations. For Ecol Manag 305:273–281. https://doi.org/10.1016/j.foreco.2013.06.003
Stemmelen A, Jactel H, Brockerhoff E, Castagneyrol B (2022) Meta-analysis of tree diversity effects on the abundance, diversity and activity of herbivores’ enemies. Basic Appl Ecol 58:130–138. https://doi.org/10.1016/j.baae.2021.12.003
Stephen FM, Dahlsten DL (1976) The arrival sequence of the arthropod complex following attack by Dendroctonus brevicomis (Coleoptera:Scolytidae) in ponderosa pine. Can Entomol 108:283–304. https://doi.org/10.4039/Ent108283-3
Stereńczak K, Mielcarek M, Modzelewska A, Kraszewski B, Fassnacht FE, Hilszczański J (2019) Intra-annual Ips typographus outbreak monitoring using a multi-temporal GIS analysis based on hyperspectral and ALS data in the Białowieża Forests. For Ecol Manag 442:105–116. https://doi.org/10.1016/j.foreco.2019.03.064
Sun D, Guo Z, Liu Y, Zhang Y (2017) Progress and prospects of CRISPR/Cas systems in insects and other arthropods. Front Physiol 8:608. https://doi.org/10.3389/fphys.2017.00608
Svensson L (2007) Övervakning av insektsangrepp:-slutrapport från Skogsstyrelsens regeringsuppdrag. Skogsstyrelsens förlag. (in Swedish)
Tam M, Capon T, Whitten S, Tapsuwan S, Kandulu J, Measham P (2023) Assessing the economic benefit of area wide management and the sterile insect technique for the Queensland fruit fly in pest-free vs. endemic regions of South-east Australia. Int J Pest Manag 69:64–80. https://doi.org/10.1080/09670874.2020.1853274
Taning CN, Mezzetti B, Kleter G, Smagghe G, Baraldi E (2021) Does RNAi-based technology fit within EU sustainability goals? Trends Biotechnol 39:644–647. https://doi.org/10.1016/j.tibtech.2020.11.008
Távora TPK, dos Santos Diniz FDA, de Moraes R-M, Freitas NC, Arraes FBM, de Andrade EC et al (2022) CRISPR/Cas-and topical RNAi-based technologies for crop management and improvement: reviewing the risk assessment and challenges towards a more sustainable agriculture. Front Bioeng Biotechnol 10:913728. https://doi.org/10.3389/fbioe.2022.913728
Thomas A, Kolb T, Biederman J, Venturas MD, Ma Q, Yang D, Dore S, Tai X (2024) Mitigating drought mortality by incorporating topography into variable forest thinning strategies. Environ Res Lett 19:034035. https://doi.org/10.1088/1748-9326/ad29aa
Trubin A (2024) The use of remote sensing imagery for the estimation of tree health characteristics related to bark beetle attack (PhD Thesis). Česká zemědělská univerzita v Praze
Trubin A, Kozhoridze G, Zabihi K, Modlinger R, Singh VV, Surový P, Jakuš R (2023) Detection of susceptible Norway spruce to bark beetle attack using PlanetScope multispectral imagery. Front for Glob Change 6:1130721. https://doi.org/10.3389/ffgc.2023.1130721
Trubin A, Kozhoridze G, Zabihi K, Modlinger R, Singh VV, Surový P, Jakuš R (2024) Detection of green attack and bark beetle susceptibility in Norway spruce: utilizing PlanetScope multispectral imagery for tri-stage spectral separability analysis. For Ecol Manag 560:121838. https://doi.org/10.1016/j.foreco.2024.121838
Turčáni M, Vakula J (2007) The influence of irradiation on the behaviour and reproduction success of eight toothed bark beetle Ips typographus (Coleoptera: Scolytidae). J for Sci 53:31–37. https://doi.org/10.17221/2154-JFS
Ulrich H (2004) Predation by adult Dolichopodidae (Diptera): a review of literature with an annotated prey-predator list. Studia Dipterologica. 11(2):369–403
Upadhyay SK (ed) (2021) Genome engineering for crop improvement. Wiley, pp 1–394. https://doi.org/10.1002/9781119672425
Vatanparast M, Park Y (2022) Cold tolerance strategies of the fall armyworm, Spodoptera frugiperda (Smith)(Lepidoptera: Noctuidae). Sci Rep 12:4129. https://doi.org/10.1038/s41598-022-08174-4
Vega FE, Hofstetter RW (eds) (2015) Bark beetles: biology and ecology of native and invasive species. Academic Press. https://doi.org/10.1016/B978-0-12-417156-5.00007-1
Vošvrdová N, Johansson A, Turčáni M, Jakuš R, Tyšer D, Schlyter F, Modlinger R (2023) Dogs trained to recognise a bark beetle pheromone locate recently attacked spruces better than human experts. For Ecol Manag 528:120626. https://doi.org/10.1016/j.foreco.2022.120626
Walter JA, Platt RV (2013) Multi-temporal analysis reveals that predictors of mountain pine beetle infestation change during outbreak cycles. For Ecol Manag 302:308–318. https://doi.org/10.1016/j.foreco.2013.03.038
Wegensteiner R (2007) Pathogens in bark beetles. In: Lieutier F, Day KR, Battisti A, Gregoire JC, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-2241-8_12
Wegensteiner R, Wermelinger B, Herrmann M (2015) Natural enemies of bark beetles: predators, parasitoids, pathogens, and nematodes. In: Vega FE, Hofstetter RW (eds) Bark beetles: biology and ecology of native and invasive species. Elsevier Academic Press, Cambridge, pp 247–304. https://doi.org/10.1016/B978-0-12-417156-5.00007-1
Wermelinger B (2002) Development and distribution of predators and parasitoids during two consecutive years of an Ips typographus (Col., Scolytidae) infestation. J Appl Entomol 126:521–527. https://doi.org/10.1046/j.1439-0418.2002.00707.x
Wermelinger B (2004) Ecology and management of the spruce bark beetle Ips typographus—a review of recent research. For Ecol Manag 202:67–82. https://doi.org/10.1016/j.foreco.2004.07.018
Wermelinger B, Epper C, Kenis M, Ghosh S, Holdenrieder O (2012) Emergence patterns of univoltine and bivoltine Ips typographus (L.) populations and associated natural enemies. J Appl Entomol 136:212–224. https://doi.org/10.1111/j.1439-0418.2011.01629.x
Wermelinger B, Obrist MK, Baur H, Jakoby O, Duelli P (2013) Synchronous rise and fall of bark beetle and parasitoid populations in windthrow areas. Agric for Entomol 15:301–309. https://doi.org/10.1111/afe.12018
West PW (2014) Growing plantation forests. Springer-Verlag. https://doi.org/10.1007/978-3-319-01827-0
White JC, Coops NC, Hilker T, Wulder MA, Carroll AL (2007) Detecting mountain pine beetle red attack damage with EO-1 Hyperion moisture indices. Int J Remote Sens 28:2111–2121. https://doi.org/10.1080/01431160600944028
Wiedenheft B, Sternberg SH, Doudna JA (2012) RNA-guided genetic silencing systems in bacteria and archaea. Nature 482:331–338. https://doi.org/10.1038/nature10886
Xu L, Lou Q, Cheng C, Lu M, Sun J (2015) Gut-associated bacteria of Dendroctonus valens and their involvement in verbenone production. Microb Ecol 70:1012–1023. https://doi.org/10.1007/s00248-015-0625-4
Yan G, Yang J, Li W, Guo A, Guan J, Liu Y (2023) Genome-wide CRISPR screens identify ILF3 as a mediator of mTORC1-dependent amino acid sensing. Nat Cell Biol 25:754–764. https://doi.org/10.1038/s41556-023-01123-x
Yoon JS, Shukla JN, Gong ZJ, Mogilicherla K, Palli SR (2016) RNA interference in the Colorado potato beetle, Leptinotarsa decemlineata: identification of key contributors. Insect Biochem Mol Biol 78:78–88. https://doi.org/10.1016/j.ibmb.2016.09.002
Yoon JS, Mogilicherla K, Gurusamy D, Chen X, Chereddy SC, Palli SR (2018) Double-stranded RNA binding protein, Staufen, is required for the initiation of RNAi in coleopteran insects. Proc Natl Acad Sci 115:8334–8339. https://doi.org/10.1073/pnas.1809381115
Young DJN, Stevens JT, Earles JM, Moore J, Ellis A, Jirka AL, Latimer AM (2017) Long-term climate and competition explain forest mortality patterns under extreme drought. Ecol Lett 20:78–86. https://doi.org/10.1111/ele.12711
Zabihi K, Surovy P, Trubin A, Singh VV, Jakuš R (2021) A review of major factors influencing the accuracy of mapping green-attack stage of bark beetle infestations using satellite imagery: prospects to avoid data redundancy. Remote Sens Appl Soc Environ 24:100638. https://doi.org/10.1016/j.rsase.2021.100638
Zabihi K, Singh VV, Trubin A, Tomášková I, Blaženec M, Surový P, Jakuš R (2023) Sap flow as a function of variables within nested scales: ordinary least squares versus spatial regression models. Environ Res Ecol. https://doi.org/10.1088/2752-664X/acd6ff
Zacharias M, Pampuch T, Dauphin B, Opgenoorth L, Roland C, Schnittler M, Wilmking M, Bog M, Heer K (2022) Genetic basis of growth reaction to drought stress differs in contrasting high-latitude treeline ecotones of a widespread conifer. Mol Ecol 31:5165–5181. https://doi.org/10.1111/mec.16648
Zhang QH, Schlyter F (2003) Redundancy, synergism, and active inhibitory range of non-host volatiles in reducing pheromone attraction in European spruce bark beetle Ips typographus. Oikos 101:299–310. https://doi.org/10.1034/j.1600-0706.2003.111595.x
Zhang QH, Birgersson G, Zhu J, Löfstedt C, Löfqvist J, Schlyter F (1999) Leaf volatiles from nonhost deciduous trees: variation by tree species, season and temperature, and electrophysiological activity in Ips typographus. J Chem Ecol 25:1923–1943. https://doi.org/10.1023/A:1020994119019
Zhang J, Ritchie MW, Maguire DA, Oliver WW (2013) Thinning ponderosa pine (Pinus ponderosa) stands reduces mortality while maintaining stand productivity. Can J for Res 43:311–320. https://doi.org/10.1139/cjfr-2012-0411
Zhang J, Huang S, He F (2015) Half-century evidence from western Canada shows forest dynamics are primarily driven by competition followed by climate. PNAS 112:4009–4014. https://doi.org/10.1073/pnas.1420844112
Zhang J, Zhao J, Cheng R, Ge Z, Zhang Z (2022) Effects of neighborhood competition and stand structure on the productivity of pure and mixed Larix principis-rupprechtii Forests. Forests 13:1318. https://doi.org/10.3390/f13081318
Zhao S, Erbilgin N (2019) Larger resin ducts are linked to the survival of lodgepole pine trees during mountain pine beetle outbreak. Front Plant Sci 10:1459. https://doi.org/10.3389/fpls.2019.01459
Zhao S, Klutsch JG, Cale JA, Erbilgin N (2019a) Mountain pine beetle outbreak enhanced resin duct-defenses of lodgepole pine trees. For Ecol Manag 441:271–279. https://doi.org/10.1016/j.foreco.2019.03.023
Zhao T, Kandasamy D, Krokene P, Chen J, Gershenzon J, Hammerbacher A (2019b) Fungal associates of the tree-killing bark beetle, Ips typographus, vary in virulence, ability to degrade conifer phenolics and influence bark beetle tunneling behavior. Fungal Ecol 38:71–79. https://doi.org/10.1016/j.funeco.2018.06.003
Zhu KY, Palli SR (2020) Mechanisms, applications, and challenges of insect RNA interference. Annu Rev Entomol 65:293–311. https://doi.org/10.1146/annurev-ento-011019-025224
Zimová S, Dobor L, Hlásny T, Rammer W, Seidl R (2020) Reducing rotation age to address increasing disturbances in Central Europe: potential and limitations. For Ecol Manag 475:118408. https://doi.org/10.1016/j.foreco.2020.118408
Acknowledgments
We thank Dr. Jiří Synek for providing beetle pictures. We also thank the editor and anonymous reviewers for their constructive comments and suggestions.
Funding
Open access publishing supported by the National Technical Library in Prague. This work was funded by Grant No. CZ.02.1.01/0.0/0.0/15_003/0000433, “EXTEMIT—K Project,” Operational Program Research, Development and Education (OP RDE), and Internal Grant Agency (Grant No. 43950/1312/3123) at the Faculty of Forestry and Wood Sciences, Czech University of Life Sciences, Prague, Czech Republic. KM and AR were supported by “Excellent Team Grant” at the Faculty of Forestry and Wood Sciences, Czech University of Life Sciences, Prague, Czech Republic. KM acknowledges the Ramalingaswami re-entry grant (BT/RLF/Re-entry/11/2023 from the Department of Biotechnology, Govt. of India.
Author information
Authors and Affiliations
Contributions
VVS and NE conceptualized the article. VVS searched the literature and wrote the first draft of the manuscript. AN, KM, AT, and KZ wrote sections of the manuscript. VVS, AN, and KZ prepared figures. KM, AR, RJ, and NE supervised the work. All authors contributed to reviewing and editing the article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
All authors gave informed consent to this publication and its content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, V.V., Naseer, A., Mogilicherla, K. et al. Understanding bark beetle outbreaks: exploring the impact of changing temperature regimes, droughts, forest structure, and prospects for future forest pest management. Rev Environ Sci Biotechnol (2024). https://doi.org/10.1007/s11157-024-09692-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11157-024-09692-5