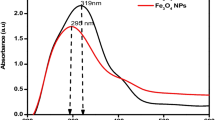
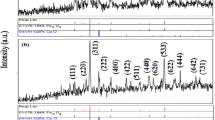
Abstract
Using piezoelectric nanoparticles created with natural surfactants to remove dyes from water is a promising, environmentally friendly approach with potential benefits in sustainability, efficiency, cost-effectiveness, and innovation in water treatment technologies. This study investigates the influence of natural surfactants on the synthesis of hydrothermal-based Ni ferrite nanoparticles designed for dye removal. Three fruit juices—grape, orange, and peach—were employed in the preparation process, and their properties were scrutinized through X-ray diffraction (XRD), scanning electron microscope (SEM), and Energy-dispersive X-ray spectroscopy (EDAX). The research also explores the effects of varying time and temperature parameters on dye removal. Results indicate that Ni-ferrite nanoparticles synthesized with grape juice exhibit enhanced efficacy in degrading crystal violet dye. Moreover, the morphology of these nanoparticles diverges from those produced through alternative methods documented in the literature. The study’s findings suggest that the degradation of Crystal Violet (CV) by a NiFe2O4 catalyst through piezoelectric means adheres to pseudo-second-order kinetics. Thermodynamic analyses reveal that CV piezo degradation is an endothermic process. The presence of nearly spherical nanoparticles in all samples is accompanied by the remarkable identification of bar-shaped crystalline particles with piezoelectric properties with a length of around 3 µm and a diameter of 300 nm in samples synthesized with grape juice. This unique morphological characteristic, which has not been previously reported for nickel ferrite, represents a novel finding. In conclusion, we posit that natural surfactants, exemplified by grape juice, exert a substantial influence on the microstructure of nanoparticles, thereby influencing their potential applications.
Graphical abstract







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Iraqui S, Kashyap SS, Rashid MH (2020) NiFe2O4 nanoparticles: an efficient and reusable catalyst for the selective oxidation of benzyl alcohol to benzaldehyde under mild conditions. Nanoscale Adv 2(12):5790–5802
Hariharasuthan R et al (2022) Characterization of NiFe2O4 (nickel ferrite) nanoparticles with very low magnetic saturation synthesized via co-precipitation method. Appl Phys A 128(12):1045
Shokri A (2021) Using NiFe2O4 as a nano photocatalyst for degradation of polyvinyl alcohol in synthetic wastewater. Environ Chall 5:100332
Fathy MA, Kamel AH, Hassan SSM (2022) Novel magnetic nickel ferrite nanoparticles modified with poly(aniline-co-o-toluidine) for the removal of hazardous 2,4-dichlorophenol pollutant from aqueous solutions. RSC Adv 12(12):7433–7445
Babu Naidu KC, Madhuri W (2017) Hydrothermal synthesis of NiFe2O4 nano-particles: structural, morphological, optical, electrical and magnetic properties. Bull Mater Sci 40(2):417–425
Kesavan G et al (2020) Hydrothermal synthesis of NiFe2O4 nanoparticles as an efficient electrocatalyst for the electrochemical detection of bisphenol A. New J Chem 44(19):7698–7707
Song T et al (2021) A review of the role and mechanism of surfactants in the morphology control of metal nanoparticles. Nanoscale 13(7):3895–3910
Naikoo GA et al (2021) Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: a critical review. J Saudi Chem Soc 25(9):101304
Aslam R et al (2023) Biosurfactants: types, sources, and production. In: Aslam R et al (eds) Advancements in biosurfactants research. Springer International Publishing, Cham, pp 3–24
Sharma J, Sundar D, Srivastava P (2023) Advantages and disadvantages of biosurfactants over other synthetic surfactants. In: Aslam R et al (eds) Advancements in biosurfactants research. Springer International Publishing, Cham, pp 505–523
Mahmoodi NM (2013) Nickel ferrite nanoparticle: synthesis, modification by surfactant and dye removal ability. Water Air Soil Pollut 224(2):1419
Zeng S et al (2014) Magnetically separable Ni0.6Fe2.4O4 nanoparticles as an effective adsorbent for dye removal: synthesis and study on the kinetic and thermodynamic behaviors for dye adsorption. Chem Eng J 258:218–228
Riyat MRI et al (2022) Magnetically recyclable core–shell structured Co0.5Zn0.5Fe2O4@polyaniline nanocomposite: high stability and rapid photocatalytic degradation of commercial azo dyes and industrial effluents. Reac Kinet Mech Cat 135(2):1077–1098
Chandra D et al (2023) Chitosan-based nano-sorbents: synthesis, surface modification, characterisation and application in Cd(II), Co(II), Cu(II) and Pb(II) ions removal from wastewater. Sci Rep 13(1):6050
Bashar MA et al (2023) Hydrothermal synthesis of cobalt substitute zinc-ferrite (Co1−xZnxFe2O4) nanodot, functionalised by polyaniline with enhanced photocatalytic activity under visible light irradiation. Heliyon 9(4):e15381
Arumugham N et al (2022) Nickel ferrite-based composites and its photocatalytic application—a review. J Hazard Mater Adv 8:100156
Bayahia H (2022) High activity of ZnFe2O4 nanoparticles for photodegradation of crystal violet dye solution in the presence of sunlight. J Taibah Univ Sci 16(1):988–1004
Alizadeh N, Mahjoub M (2017) Removal of crystal violet dye from aqueous solution using surfactant modified NiFe2O4 as nanoadsorbent; isotherms, thermodynamics and kinetics studies. J Nanoanal 4(1):8–19
Aspoukeh PK, Barzinjy AA, Hamad SM (2023) A novel approach to the green synthesis of zinc oxide nanorods using Thymus kotschyanus plant extract: effect of ammonium hydroxide and precursor concentration. Nano Express. https://doi.org/10.1088/2632-959X/acfe25
Vinaykumar R et al (2021) Synthesis and characterization of Ba2Co2Fe12O22–NiFe2O4 ferrite composites: a useful substrate material in miniaturizing antenna. J Mater Sci Mater Electron 32(6):7330–7339
Mandizadeh S, Amiri O, Salavati-Niasari M (2021) Effects of the NiFe2O4 nanoadditive on the performance and emission characteristics of diesel engines: ultrasonic green synthesis by T3 hormone. RSC Adv 11(44):27701–27713
Cherpin C et al (2021) Study of the solid-state synthesis of nickel ferrite (NiFe2O4) by X-ray diffraction (XRD), scanning electron microscopy (SEM) and Raman spectroscopy. Materials. https://doi.org/10.3390/ma14102557
Hariharasuthan R et al (2022) Characterization of NiFe2O4 (nickel ferrite) nanoparticles with very low magnetic saturation synthesized via co-precipitation method. Appl Phys A 128(12):1045٧
Sapna et al (2017) X-ray analysis of NiFe2O4 nanoparticles by Williamson-Hall and size-strain plot method. J Adv Phys 6(4):492–495
Sivakumar P et al (2011) Synthesis and characterization of nickel ferrite magnetic nanoparticles. Mater Res Bull 46(12):2208–2211
Lafta SH (2017) Effect of pH on structural, magnetic and FMR properties of hydrothermally prepared nano Ni ferrite. Open Chem 15(1):53–60
Srivastava M, Chaubey S, Ojha AK (2009) Investigation on size dependent structural and magnetic behavior of nickel ferrite nanoparticles prepared by sol–gel and hydrothermal methods. Mater Chem Phys 118(1):174–180
Majid F et al (2021) Synthesis and characterization of NiFe2O4 ferrite: sol–gel and hydrothermal synthesis routes effect on magnetic, structural and dielectric characteristics. Mater Chem Phys 258:123888
Babakr KA et al (2022) Kinetic and thermodynamic study in piezo degradation of methylene blue by SbSI/Sb2S3 nanocomposites stimulated by zirconium oxide balls. Sci Rep 12(1):15242
Zuo X et al (2006) A computational study of nickel ferrite. J Magn Magn Mater 303(2):e432–e435
Srivastava M et al (2009) Synthesis and optical characterization of nanocrystalline NiFe2O4 structures. J Alloys Compd 481(1):515–519
Sen R et al (2015) Synthesis and characterization of nickel ferrite (NiFe2O4) nanoparticles prepared by sol-gel method. Mater Today Proc 2(4):3750–3757
Rajamanickam D, Shanthi M (2016) Photocatalytic degradation of an organic pollutant by zinc oxide—solar process. Arab J Chem 9:S1858–S1868
Moussout H, Ahlafi H, Aazza M, Maghat H (2018) Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala Int J Mod Sci 4(2):244–254. https://doi.org/10.1016/j.kijoms.2018.04.001
Babakr KA, Aziz BK (2019) Adsorptive removal of methyl orange from aqueous solutions with natural Garmak clay as cheap and efficient adsorbent in batch and continuous systems. J Zankoy Sulaimani A 21(2):183–200
Aziz BK, Karim MAH (2019) Efficient catalytic photodegradation of methylene blue from medical lab wastewater using MgO nanoparticles synthesized by direct precipitation method. Reac Kinet Mech Cat 128:1127–1139
Sandhu ZA et al (2023) Response surface methodology: a powerful tool for optimizing the synthesis of metal sulfide nanoparticles for dye degradation. Mater Adv 4(21):5094–5125
Kaveh R et al (2024) Ternary nanohybrid of biochar/NiFe2O4/Ag3PO4 for simultaneous adsorption of Hg(II) and photodegradation of methylene blue; modeling, kinetic and isotherm studies. J Solid State Chem 331:124503
Rana G et al (2021) Recent advances on nickel nano-ferrite: a review on processing techniques, properties and diverse applications. Chem Eng Res Des 175:182–208
Acknowledgements
The authors are appreciative of the financial support they received from the University of Raparin and Soran University—Scientific Research Center.
Author information
Authors and Affiliations
Contributions
O. Amiri supervised the work and Review. K. A. Babakr and I. N. Qader Sample preparation, Experimental Design, and Writing manuscript. M. Özabaci, P. Aspoukeh, and S. M. Hamad Characterization.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Babakr, K.A., Qader, I.N., Amiri, O. et al. Synthesis and characterization of NiFe2O4 piezoelectric nanoparticles: a comprehensive study on the influence of natural surfactants, kinetics, and thermodynamics. Reac Kinet Mech Cat (2024). https://doi.org/10.1007/s11144-024-02607-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11144-024-02607-z