Abstract
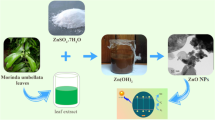
Green synthesis–based metal oxide nanoparticle fabrication is an intriguing topic. The synthesis of MgO nanoparticles (S1, S2, S3) was achieved using the extracts of Madhuca longifolia flowers. Subsequent research revealed that the physical morphology of the nanoparticles was improved as the concentration of the reducing agent (E1, E2, E3) increased. The nanoparticles were then employed for the removal of nigrosine dye (a common water contaminant) through adsorption and photocatalysis. The dye removal process was optimized using the TAGUCHI L25 array from MINITAB software. Among the morphologically improved samples, S3 exhibited the most notable performance in terms of % dye clearance, with a remarkable rate of 99%. S3 was then employed in the investigation of adsorption kinetics and isotherms. The obtained results indicate that the model exhibits a strong match to both the pseudo-second-order model and the Langmuir model, as evidenced by the high R2 value of 0.99 in both cases. Consequently, the Langmuir model can be considered the most suitable choice. This study demonstrates that environmentally sustainable techniques have the capacity to modify the morphology of nanoparticles. Furthermore, the enhancements in morphology have demonstrated significant effectiveness in their intended application. The utilization of MgO nanoparticles in wastewater treatment is supported by their non-toxic nature, sustainable characteristics, and the implementation of straightforward, cost-effective, and efficient manufacturing techniques.









Similar content being viewed by others

Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- MgO:
-
Magnesium oxide
- M. longifolia :
-
Madhuca longifolia
- UV:
-
Ultraviolet
- XRD:
-
X-ray diffraction analysis
- FTIR:
-
Fourier transform infrared spectroscopy
- FESEM:
-
Field emission scanning electron microscopy
- E:
-
Extract
- S:
-
Sample
- %:
-
Percent
- SPR:
-
Surface plasmon resonance
- nm:
-
Nanometer
- DOE:
-
Design of experiments
- R 2 :
-
Regression coefficients
References
Gupta VK, Jain R, Nayak A, Agarwal S, Shrivastava M (2011) Removal of the hazardous dye-tartrazine by photodegradation on titanium dioxide surface. Mater Sci Eng C 31(5):1062–1067. https://doi.org/10.1016/j.msec.2011.03.006
Berradi M et al (2019) Textile finishing dyes and their impact on aquatic environs. Heliyon 5(11). https://doi.org/10.1016/j.heliyon.2019.e02711
Louati I et al (2020) Simultaneous cleanup of Reactive Black 5 and cadmium by a desert soil bacterium. Ecotoxicol Environ Saf 190:110103. https://doi.org/10.1016/j.ecoenv.2019.110103
Yang Q et al (2018) Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem Eng J 333:49–57. https://doi.org/10.1016/j.cej.2017.09.099
Othmani A, Kesraoui A, Seffen M (2017) The alternating and direct current effect on the elimination of cationic and anionic dye from aqueous solutions by electrocoagulation and coagulation flocculation. EuroMediterr J Environ Integr 2(1). https://doi.org/10.1007/s41207-017-0016-y
R. Mia et al., “Review on various types of pollution problem in textile dyeing & printing industries of Bangladesh and recommandation for mitigation,” J Text Eng Fash Technol, 5, 4, Aug. 2019, https://doi.org/10.15406/jteft.2019.05.00205.
Byrne C, Subramanian G, Pillai SC (2018) Recent advances in photocatalysis for environmental applications. J Environ Chem Eng 6(3):3531–3555. https://doi.org/10.1016/j.jece.2017.07.080
Othmani A, Kesraoui A, Akrout H, López-Mesas M, Seffen M, Valiente M (2019) Use of alternating current for colored water purification by anodic oxidation with SS/PbO2 and Pb/PbO2 electrodes. Environ Sci Pollut Res 26(25):25969–25984. https://doi.org/10.1007/s11356-019-05722-w
Ding Y et al (2023) Relay photo/thermal catalysis enables efficient cascade upgrading of sugars to lactic acid: mechanism study and life cycle assessment. Chem Eng J 452:139687. https://doi.org/10.1016/j.cej.2022.139687
Xiong L, Sun W, Yang Y, Chen C, Ni J (2011) Heterogeneous photocatalysis of methylene blue over titanate nanotubes: effect of adsorption. J Colloid Interface Sci 356(1):211–216. https://doi.org/10.1016/j.jcis.2010.12.059
N. Tara, S. I. Siddiqui, G. Rathi, S. A. Chaudhry, Inamuddin, and A. M. Asiri, “Nano-engineered adsorbent for the removal of dyes from water: a review,” Curr Anal Chem, 16, 1, 14–40, 2019, https://doi.org/10.2174/1573411015666190117124344.
Chan SHS, Wu TY, Juan JC, Teh CY (2011) Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J Chem Technol Biotechnol 86(9):1130–1158. https://doi.org/10.1002/jctb.2636
Yue C et al (2023) Metal–organic framework-based materials: emerging high-efficiency catalysts for the heterogeneous photocatalytic degradation of pollutants in water. Environ Sci (Camb) 9(3):669–695. https://doi.org/10.1039/D2EW00784C
Tayeb AM, Hussein DS (2015) Synthesis of TiO 2 Nanoparticles and their photocatalytic activity for methylene blue. Am J Nanomater 3(2):57–63. https://doi.org/10.12691/ajn-3-2-2
Bagheri M, Najafabadi NR, Borna E (2020) Removal of reactive blue 203 dye photocatalytic using ZnO nanoparticles stabilized on functionalized MWCNTs. J King Saud Univ Sci 32(1):799–804. https://doi.org/10.1016/J.JKSUS.2019.02.012
Afsharpour M, Elyasi M, Javadian H (2021) A novel N-doped nanoporous bio-graphene synthesized from Pistacia lentiscus gum and its nanocomposite with WO3 nanoparticles: visible-light-driven photocatalytic activity. Molecules 26(21):6569. https://doi.org/10.3390/molecules26216569
Haseena S et al (2022) Bio-synthesize of photocatalytic Fe2O3 nanoparticles using Leucas aspera and Jatropha podagrica leaf extract for an effective removal of textile dye pollutants. Optik (Stuttg) 249:168275. https://doi.org/10.1016/J.IJLEO.2021.168275
Moussavi G, Mahmoudi M (2009) Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J Hazard Mater 168(2–3):806–812. https://doi.org/10.1016/J.JHAZMAT.2009.02.097
Sibhatu AK, Weldegebrieal GK, Sagadevan S, Tran NN, Hessel V (2022) Photocatalytic activity of CuO nanoparticles for organic and inorganic pollutants removal in wastewater remediation. Chemosphere 300:134623. https://doi.org/10.1016/J.CHEMOSPHERE.2022.134623
Sahoo U, Pattnayak S, Choudhury S, Aparajita P, Pradhan DK, Hota G (2023) Facile synthesis of defect induced CeO2/MIL-53(Fe) nanocatalyst: strategically switching the charge transfer dynamics for remarkable enhancement of photocatalytic bisphenol A DEGRADATION and H2 evolution. Appl Catal B:123524. https://doi.org/10.1016/j.apcatb.2023.123524
Pachiyappan J, Gnanansundaram N, Sivamani S, Sankari NPBP, Senthilnathan N, Kerga GA (2022) Preparation and characterization of magnesium oxide nanoparticles and its application for photocatalytic removal of rhodamine B and methylene blue dyes. J Nanomater 2022. https://doi.org/10.1155/2022/6484573
Jorfi S et al (2016) Enhanced coagulation-photocatalytic treatment of Acid red 73 dye and real textile wastewater using UVA/synthesized MgO nanoparticles. J Environ Manage 177:111–118. https://doi.org/10.1016/j.jenvman.2016.04.005
Ge L et al (2018) Facile fabrication of Fe@MgO magnetic nanocomposites for efficient removal of heavy metal ion and dye from water. Powder Technol 326:393–401. https://doi.org/10.1016/j.powtec.2017.12.003
Cao C-Y, Qu J, Wei F, Liu H, Song W-G (2012) Superb adsorption capacity and mechanism of flowerlike magnesium oxide nanostructures for lead and cadmium ions. ACS Appl Mater Interfaces 4(8):4283–4287. https://doi.org/10.1021/am300972z
Ramezani Farani M, Farsadrooh M, Zare I, Gholami A, Akhavan O (2023) Green synthesis of magnesium oxide nanoparticles and nanocomposites for photocatalytic antimicrobial, antibiofilm and antifungal applications. Catalysts 13(4):642. https://doi.org/10.3390/catal13040642
Kumar SA et al (2022) Facile green synthesis of magnesium oxide nanoparticles using tea (Camellia sinensis) extract for efficient photocatalytic degradation of methylene blue dye. Environ Technol Innov 28:102746. https://doi.org/10.1016/j.eti.2022.102746
Khan F et al (2022) Prospects of algae-based green synthesis of nanoparticles for environmental applications. Chemosphere 293:133571. https://doi.org/10.1016/j.chemosphere.2022.133571
Nejati M et al (2022) Green methods for the preparation of MgO nanomaterials and their drug delivery, anti-cancer and anti-bacterial potentials: a review. Inorg Chem Commun 136:109107. https://doi.org/10.1016/j.inoche.2021.109107
A. K. K. Shobham, J. Panwar, and S. Gupta, “One-pot synthesis of metal oxide-clay composite for the evaluation of dye removal studies: Taguchi optimization of parameters and environmental toxicity studies,” Environ Sci Pollut Res, 30, 22, 61541–61561, Oct. 2022, https://doi.org/10.1007/s11356-022-23752-9.
Okçu GD, Tunacan T, Dikmen E (2019) Removal of indigo dye by photocatalysis process using Taguchi experimental design. Environ.Sci Technol 2(2):63–72. https://doi.org/10.35208/ert.457739
Yusuff AS, Ajayi OA, Popoola LT (2021) Application of Taguchi design approach to parametric optimization of adsorption of crystal violet dye by activated carbon from poultry litter. Sci Afr 13:e00850. https://doi.org/10.1016/j.sciaf.2021.e00850
Singh V, Singh J, Kushwaha R, Singh M, Kumar S, Rai AK (2020) Assessment of antioxidant activity, minerals and chemical constituents of edible mahua (Madhuca longifolia) flower and fruit of using principal component analysis. Nutr Food Sci 51(2):387–411. https://doi.org/10.1108/NFS-04-2020-0129
K. Mageshwari, S. S. Mali, R. Sathyamoorthy, and P. S. Patil, “Template-free synthesis of MgO nanoparticles for effective photocatalytic applications,” Powder Technol, 249, 456–462, Nov. 2013, https://doi.org/10.1016/j.powtec.2013.09.016.
Rajagopal S, Paramasivam B, Muniyasamy K (2020) Photocatalytic removal of cationic and anionic dyes in the textile wastewater by H2O2 assisted TiO2 and micro-cellulose composites. Sep Purif Technol 252:117444. https://doi.org/10.1016/j.seppur.2020.117444
Robati D (2013) Pseudo-second-orders kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J Nanostructure Chem 3(1):1–6. https://doi.org/10.1186/2193-8865-3-55
Lajevardi A, Hossaini Sadr M, Tavakkoli Yaraki M, Badiei A, Armaghan M (2018) A pH-responsive and magnetic Fe3O4@silica@MIL-100(Fe)/β-CD nanocomposite as a drug nanocarrier: Loading and release study of cephalexin. New J Chem 42(12):9690–9701. https://doi.org/10.1039/c8nj01375f
Tran HN, Lima EC, Juang R-S, Bollinger J-C, Chao H-P (2021) Thermodynamic parameters of liquid–phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. J Environ Chem Eng 9(6):106674. https://doi.org/10.1016/j.jece.2021.106674
Junghare K, Kodape S, Jadhao V (2019) Adsorption study of F-ions onto ultrasonified electrochemically generated ultrafine particles. Desalination Water Treat. https://doi.org/10.5004/dwt.2019.24773
Choudhury R, Kodape SM, Bansod PG (2022) Removal of nigrosine by MgO nanoparticles, green synthesized using Madhuca longifolia flower extract. Environ Dev Sustain 24(5):6413–6434. https://doi.org/10.1007/s10668-021-01708-2
Nemade KR, Waghuley SA (2014) Synthesis of MgO nanoparticles by solvent mixed spray pyrolysis technique for optical investigation. Int J Met 2014(3):1–4. https://doi.org/10.1155/2014/389416
Tharani K, Jegatha Christy A, Sagadevan S, Nehru LC Fabrication of magnesium oxide nanoparticles using combustion method for a biological and environmental cause. Chem Phys Lett 763:138216. https://doi.org/10.1016/j.cplett.2020.138216
Bhadwal AS, Tripathi RM, Gupta RK, Kumar N, Singh RP, Shrivastav A (2014) Biogenic synthesis and photocatalytic activity of CdS nanoparticles. RSC Adv 4(19):9484–9490. https://doi.org/10.1039/c3ra46221h
Imani MM, Safaei M (2019) Optimized synthesis of magnesium oxide nanoparticles as bactericidal agents. J Nanotechnol 2019. https://doi.org/10.1155/2019/6063832
Bhagath Singh GVP, Sonat C, Yang EH, Unluer C (2020) Performance of MgO and MgO–SiO2 systems containing seeds under different curing conditions. Cem Concr Compos 108:103543. https://doi.org/10.1016/j.cemconcomp.2020.103543
Bindhu MR, Umadevi M, Kavin Micheal M, Arasu MV, Abdullah Al-Dhabi N (2016) Structural, morphological and optical properties of MgO nanoparticles for antibacterial applications. Mater Lett 166:19–22. https://doi.org/10.1016/j.matlet.2015.12.020
Balakrishnan G, Velavan R, Mujasam Batoo K, Raslan EH (2020) Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys 16:103013. https://doi.org/10.1016/j.rinp.2020.103013
Park BK, Jeong S, Kim D, Moon J, Lim S, Kim JS (2007) Synthesis and size control of monodisperse copper nanoparticles by polyol method. J Colloid Interface Sci 311(2):417–424. https://doi.org/10.1016/j.jcis.2007.03.039
Panimalar S et al (2022) Effect of Ag doped MnO2 nanostructures suitable for wastewater treatment and other environmental pollutant applications. Environ Res 205:112560. https://doi.org/10.1016/j.envres.2021.112560
Askari MB, Rozati SM, Salarizadeh P, Saeidfirozeh H, Di Bartolomeo A (2021) A remarkable three-component RuO2-MnCo2O4/rGO nanocatalyst towards methanol electrooxidation. Int J Hydrogen Energy 46(74):36792–36800. https://doi.org/10.1016/j.ijhydene.2021.08.207
Jahanshahi M, Sanati MH, Babaei Z (2008) Optimization of parameters for the fabrication of gelatin nanoparticles by the Taguchi robust design method. J Appl Stat 35(12):1345–1353. https://doi.org/10.1080/02664760802382426
S. Athreya and Y. D. Venkatesh, “Application of Taguchi method for optimization of process parameters in improving the surface roughness of lathe facing operation,” 2012. [Online]. Available: www.irjes.comwww.irjes.com. Accessed 20 Oct 2023
Schober P, Schwarte LA (2018) Correlation coefficients: Appropriate use and interpretation. Anesth Analg 126(5):1763–1768. https://doi.org/10.1213/ANE.0000000000002864
Muthukumaran C, Sivakumar VM, Thirumarimurugan M (2016) Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J Taiwan Inst Chem Eng 63:354–362. https://doi.org/10.1016/j.jtice.2016.03.034
Rajahmundry GK, Garlapati C, Kumar PS, Alwi RS, Vo D-VN (2021) Statistical analysis of adsorption isotherm models and its appropriate selection. Chemosphere 276:130176. https://doi.org/10.1016/j.chemosphere.2021.130176
Felix N et al (2018) Activated plantain peel biochar as adsorbent for removal of zinc(II) ions from aqueous solution: equilibrium and kinetics studies. J Turk Chem Soc Sect A: Chem 5(3):1257–1270. https://doi.org/10.18596/jotcsa.438332
Gouamid M, Ouahrani MR, Bensaci MB (2013) Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using date palm leaves. Energy Procedia 36:898–907. https://doi.org/10.1016/j.egypro.2013.07.103
Said KA et al (2018) Application of Freundlich and Temkin isotherm to study the removal of Pb(II) via adsorption on activated carbon equipped polysulfone membrane. Int J Eng Technol 7(3.18):91. https://doi.org/10.14419/ijet.v7i3.18.16683
Ranjithkumar V, Sangeetha S, Vairam S (2014) Synthesis of magnetic activated carbon/α-Fe2O3 nanocomposite and its application in the removal of acid yellow 17 dye from water. J Hazard Mater 273:127–135. https://doi.org/10.1016/j.jhazmat.2014.03.034
Author information
Authors and Affiliations
Contributions
PIK studied and worked over the general mechanism and synthesis processes of MgO nanoparticles involving green synthesis and performed the analytical tests for the samples. SM studied whether the concentration variation affects the morphology of the finished product and optimized the study using Taguchi analysis. Dr. SMK studied and worked over different methodologies and conceptualization of the entire topic. Dr. KB investigated the requisite analytical techniques for confirming successful outcomes.
All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent to publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kurhade, P.I., Mittal, S., Kodape, S.M. et al. Optimization of photocatalytic removal of nigrosine dye using green synthesized MgO nanoparticles. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05313-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05313-x