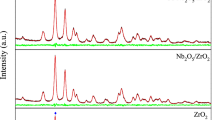
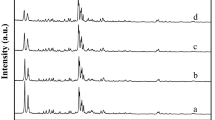
Abstract
The effect of water on the modification of acidic properties of Nb2O5 and Nb1.3MnOx catalysts was investigated using the cracking of cumene as model reaction, and compared to the behavior of a HZSM-5 catalyst. Nb1.3MnOx exhibited stronger Lewis acidity than Nb2O5, which translated into a higher selectivity towards α-methylstyrene formed on Lewis acid sites (LAS) by dehydrogenation of cumene. Steam enhanced strongly the conversion of cumene over both Nb-based catalysts. The products distribution on Nb-based catalysts was also deeply modified in the presence of steam, the selectivity towards α-methylstyrene decreasing strongly in favor of benzene, which is formed on Brønsted acid sites (BAS) by dealkylation of cumene. In contrast, the performances of HZSM-5 for cumene cracking and the products distribution were only marginally modified in the presence of steam. A kinetic model based on the elementary steps of the cumene reaction pathways (dealkylation and dehydrogenation) was used to estimate the ratio of LAS to BAS in absence and presence of water over Nb1.3MnOx. The activation energy of the cracking reaction was higher than that of the dehydrogenation reaction. The model described correctly the changes in the catalyst activity induced by addition of ≈2 V% of water, which resulted in a decrease in the [LAS]/[BAS] ratio from approximatively 3 to 1.











Similar content being viewed by others
Data availability
The data can be provided on demand.
References
Shali NB, Sugunan S (2007) Influence of transition metals on the surface acidic properties of Titania prepared by sol–gel route. Mater Res Bull. https://doi.org/10.1016/j.materresbull.2006.11.016
Corma A, Wojciechowski BW (1982) The catalytic cracking of cumene. Catal Rev-Sci Eng. https://doi.org/10.1080/03602458208079649
Guisnet M, Pinard L (2018) Characterization of acid-base catalysts through model reactions. Catal Rev. https://doi.org/10.1080/01614940.2018.1446683
Sazegar MR, Jalil AA, Triwahyono S, Mukti RR, Aziz M, Aziz MMA, Setiabudi HD, Kamarudin NHN (2014) Protonation of Al-grafted mesostructured silica nanoparticles (MSN): acidity and catalytic activity for cumene conversion. Chem Eng J. https://doi.org/10.1016/j.cej.2013.12.004
Patrick F, Sunugan S (2011) Cracking of cumene on tungsten promoted ceria catalysts. React Kinet Mech Cat. https://doi.org/10.1007/s11144-011-0309-0
Sohn JR, Ryu SG (2001) Redox and catalytic behaviors of chromium oxide supported on zirconia. Catal Lett. https://doi.org/10.1023/A:1016643232442
Parry EP (1963) An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J Catal. https://doi.org/10.1016/0021-9517(63)90102-7
Busca G (1998) Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal Today. https://doi.org/10.1016/S0920-5861(98)00049-2
Lercher JA, Gründling C, Eder-Mirth G (1996) Infrared studies of the surface acidity of oxides and zeolites using adsorbed probe molecules. Catal Today. https://doi.org/10.1016/0920-5861(95)00248-0
Yang X, Liu Z, Wei G et al (2022) A critical assessment of the roles of water molecules and solvated ions in acid-base-catalyzed reactions at solid-water interfaces. Chinese J Catal. https://doi.org/10.1016/S1872-2067(21)64032-9
Sushkevich VL, Kots PA, Kolyagin YG et al (2019) Origin of water-induced brønsted acid sites in Sn-BEA zeolites. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.8b12462
Omata K, Nambu T (2020) Catalysis of water molecules acting as Brönsted acids at Lewis acid sites on niobium oxide. Appl Catal. https://doi.org/10.1016/j.apcata.2020.117812
Kitano T, Shishido T, Teramura K, Tanaka T (2012) Brønsted acid property of alumina-supported niobium oxide calcined at high temperatures: characterization by acid-catalyzed reactions and spectroscopic methods. J Phys Chem. https://doi.org/10.1021/jp3032429
Cui Z, Feng X, Li H, Tan T (2020) Interconversion of Lewis acid and Brønsted acid catalysts in biomass-derived paraxylene synthesis. Chem Eng Sci. https://doi.org/10.1016/j.ces.2020.115942
Rahman MM, Liu R, Cai J (2018) Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—a review. Fuel Process Technol. https://doi.org/10.1016/j.fuproc.2018.08.002
Bhoi PR, Ouedraogo AS, Soloiu V, Quirino R (2020) Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew Sust Energ Rev. https://doi.org/10.1016/j.rser.2019.109676
Paasikallio V, Kalogiannis K, Lappas A et al (2017) Catalytic fast pyrolysis: influencing bio-oil quality with the catalyst-to-biomass ratio. Energy Technol. https://doi.org/10.1002/ente.201600094
Mullen CA, Boateng AA (2013) Accumulation of inorganic impurities on HZSM-5 zeolites during catalytic fast pyrolysis of switchgrass. Ind Eng Chem Res. https://doi.org/10.1021/ie4030209
Liu C, Wang H, Karim AM et al (2014) Catalytic fast pyrolysis of lignocellulosic biomass. Chem Soc Rev. https://doi.org/10.1039/C3CS60414D
Dai L, Zhou N, Li H et al (2020) Recent advances in improving lignocellulosic biomass-based bio-oil production. J Anal Appl Pyrolysis. https://doi.org/10.1016/j.jaap.2020.104845
Grams J, Jankowska A, Goscianska J (2023) Advances in design of heterogeneous catalysts for pyrolysis of lignocellulosic biomass and bio-oil upgrading. Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2023.112761
de Rezende LW, Laurenti D, Schuurman Y, Guilhaume N (2021) Ex-situ catalytic upgrading of pyrolysis vapors using mixed metal oxides. J Anal Appl Pyrolysis. https://doi.org/10.1016/j.jaap.2021.105241
Bridgwater AV (2012) Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy. https://doi.org/10.1016/j.biombioe.2011.01.048
Mostafazadeh AK, Solomatnikova O, Drogui P, Tyagi RD (2018) A review of recent research and developments in fast pyrolysis and bio-oil upgrading. Biomass Conv Bioref. https://doi.org/10.1007/s13399-018-0320-z
Zeng K, Wang Y, Huang C, Liu H, Liu X, Wang Z, Yu J, Zhang C (2021) Catalytic combustion of propane over MnNbOx composite oxides: the promotional role of niobium. Ind Eng Chem Res. https://doi.org/10.1021/acs.iecr.1c00699
Yang P, Zuo S, Chi Z, Tao F, Zhou R (2016) Elimination of 1,2-dichloroethane over (Ce, Cr)xO2/MOy catalysts (M = Ti, V Nb, Mo, W and La). Appl Catal B. https://doi.org/10.1016/j.apcatb.2016.03.017
Yang P, Fan S, Chen Z, Bao G, Zuo S, Qi C (2018) Synthesis of Nb2O5 based solid superacid materials for catalytic combustion of chlorinated VOCs. Appl Catal B. https://doi.org/10.1016/j.apcatb.2018.07.061
Busca G (1999) The surface acidity of solid oxides and its characterization by IR spectroscopic methods. An attempt at systematization. Phys Chem Chem Phys. https://doi.org/10.1039/A808366E
Yamashita K, Hirano M, Okumura K, Niwa M (2006) Activity and acidity of Nb2O5–MoO3 and Nb2O5–WO3 in the Friedel–Crafts alkylation. Catal Today. https://doi.org/10.1016/j.cattod.2006.07.025
Tang X, Li J, Sun L, Hao J (2010) Origination of N2O from NO reduction by NH3 over β-MnO2 and β-Mn2O3. Appl Catal B. https://doi.org/10.1016/j.apcatb.2010.06.012
Chau HK, Mai HD, Gumidyala A, Pham TN, Bui D-P, D’Amico AD, Alalq I, Glatzhofer DT, White JL, Crossley SP (2023) Effect of water on cumene dealkylation over H-ZSM-5 zeolites. ACS Catal. https://doi.org/10.1021/acscatal.2c05759
Lashaki MJ, Fayaz M, Niknaddaf S, Hashisho Z (2012) Effect of the adsorbate kinetic diameter on the accuracy of the Dubinin–Radushkevich equation for modeling adsorption of organic vapors on activated carbon. J Hazardous Mater. https://doi.org/10.1016/j.jhazmat.2012.09.024
Campbell DR, Wojciechowski B (1971) Catalytic cracking of cumene on aging catalysts. I. The mechanism of the reaction. J Catal. https://doi.org/10.1016/0021-9517(71)90082-0
HSC Chemistry for Windows, Outokumpu Research Oy (1999).
EUROKIN spreadsheet for assessment of transport limitations in gas-solid fixed beds https://www.eurokin.org/wp-content/uploads/webtool/EUROKIN_fixed-bed_html.htm. Accessed Feb 2023
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Indus Appl Math. https://doi.org/10.1137/0111030
Levenberg K (1944) A method for the solution of certain problems in least squares. Quart Appl Math. https://doi.org/10.1090/qam/10666
Froment GF, Bischoff KB, de Wilde J (2010) Chemical reactor analysis and design, 3rd edn. Wiley
Acknowledgements
The “Waste to Road” project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement N° 818120.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Rezende Locatel, W., Laurenti, D., Schuurman, Y. et al. Effect of steam on the modification of Brønsted/Lewis acidity of Nb–Mn mixed oxide catalysts. Reac Kinet Mech Cat 137, 251–268 (2024). https://doi.org/10.1007/s11144-023-02536-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02536-3