Abstract
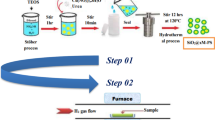
Bi- or Sb-promoted Pt catalysts with various Pt/Promoter ratios were supported on activated carbon (AC) by electroless deposition. Samples were characterized by inductively coupled plasma-optical emission spectroscopy (ICP-OES), N2 physisorption, X-ray diffractometry (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDX). The highest compositional homogeneity was found for 1%Pt–5%Bi, while 5%Pt–1%Bi has the highest platinum exposure on the surface. Pt–Sb samples consist of Pt- and Sb-containing nanochains; isolated Pt particles are only observed on a sample high in Pt and low in Sb (5%Pt–1%Sb). The suitability of the catalysts for the oxidative coupling of 2-methyl-1-naphthol depends on the promoter (Pro) used, the Pt loading, the Pt/Pro ratio, the solvent and the temperature of the reaction. 5%Pt–1%Bi/AC has the highest activity in terms of catalytic cycles completed; at room temperature in MeNO2 the binaphthol (3,3’-dimethyl-1,1’-binaphthalenyl-4,4’-diol) is obtained with an isolated yield of 99%. The highest yield (89%) of the binaphthone (3,3’-dimethyl-1,1’-binaphthalenylidene-4,4’-dione) is observed over 1%Pt-5%Bi/AC in refluxing MeOH.





Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available since it is an ongoing study, but they are obtainable from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M (2002) Chem Rev 102:1359–1469
Takeya T, Kondo H, Otsuka T, Doi H, Okamoto I, Kotani E (2005) Chem Pharm Bull 53:199–206
Adeniyi AA, Ajibade PA (2018). Bioinorg Chem Appl. https://doi.org/10.1155/2018/5796287
Otsuka T, Okamoto I, Kotani E, Takeya T (2004) Tetrahedron Lett 45:2643–2647
Ogata T, Okamoto I, Kotani E, Takeya T (2004) Tetrahedron 60:3941–3948
Takeya T, Doi H, Ogata T, Okamoto I, Kotani E (2004) Tetrahedron 60:9049–9060
Ganapaty S, Thomas PS, Fotso S, Laatsch H (2004) Phytochemistry 65:1267–1271
Jansen PCM (2005). In: Jansen PCM, Cardon D (eds) Plant Resources of Tropical Africa. Earthprint Limited, Wageningen, Netherlands
Ogata T, Okamoto I, Doi H, Kotani E, Takeya T (2003) Tetrahedron Lett 44:2041–2044
Maphoru MV, Heveling J, Kesavan Pillai S (2017) Kinet Catal 58:441–447
Maphoru MV, Kesavan Pillai S, Heveling J (2017) J Catal 348:47–58
Maphoru MV, Heveling J, Kesavan Pillai S (2018) ChemistrySelect 3:6224–6231
Besson M, Gallezot P (2000) Catal Today 57:127–141
Haan JL, Stafford KM, Masel RI (2010) J Phys Chem C 114:11665–11672
Roy K, Artiglia L, Xiao Y, Varma A, van Bokhoven JA (2019) ACS Catal 9:3694–3699
Besson M, Lahmer F, Gallezot P, Fuertes P, Flèche G (1995) J Catal 152:116–121
Mallat T, Bodnar Z, Hug P, Baiker A (1995) J Catal 153:131–143
Anderson R, Griffin K, Johnston P, Alsters PL (2003) Adv Synth Catal 345:517–523
Mallat T, Baiker A (2004) Chem Rev 104:3037–3058
Yu X, Pickup PG (2011) J Power Sources 196:7951–7956
Mallat T, Bodnar Z, Baiker A, Greis O, Strübig H, Reller A (1993) J Catal 142:237–253
Inoue T, Asakura K, Li W, Oyama T, Iwasawa Y (1997) Appl Catal A 165:183–197
Inoue T, Asakura K, Iwasawa Y (1997) J Catal 171:457–466
Campbell CT, Campbell JM, Dalton PJ, Henn FC, Rodriguez JA, Seimanides SG (1989) J Phys Chem 93:806–814
Maphoru MV, Heveling J, Pillai SK (2014) ChemPlusChem 79:99–106
Maphoru MV, Heveling J, Kesavan Pillai S (2016) Eur J Org Chem 2016(2):331–337
Geng J, Hou W-H, Lv Y-N, Zhu J-J, Chen H-Y (2005) Inorg Chem 44:8503–8509
Wang C, Shao C, Liu Y, Zhang L (2008) Scr Mater 59:332–334
Long B, Huang J, Wang X (2012) Prog Nat Sci Mater Int 22:644–653
Lee JK, Jeon H, Uhm S, Lee J (2008) Electrochim Acta 53:6089–6092
Yu X, Pickup PG (2010) Electrochim Acta 55:7354–7361
Chin HS, Cheong KY, Razak KA (2010) J Mater Sci 45:5993–6008
Barrett EP, Joyner LG, Halenda PP (1951) J Am Chem Soc 73:373–380
Acknowledgements
M.V. Maphoru gratefully acknowledges financial support by a bursary received from DAAD-NRF (Deutscher Akademischer Austauschdienst—National Research Foundation of South Africa). The authors are thankful for the assistance received from Ms. Louise Mostert for XPS analysis (National Metrology Institute of South Africa), and from Ms. Charity Maepa and Ms. Rirandzu Rikhotso for SEM and TEM analysis (Council for Scientific and Industrial Research, Pretoria).
Funding
M.V. Maphoru gratefully acknowledges financial support by a bursary received from DAAD-NRF (Deutscher Akademischer Austauschdienst—National Research Foundation of South Africa) and conference attendance fees received from Tshwane University of Technology.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and the design of the study. Material preparation, data collection and data analyses were performed by MVM and supervised by JH and SKP. The first draft of the manuscript was written by MVM, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The Authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maphoru, M.V., Heveling, J. & Kesavan Pillai, S. Impact of the promoter on the performance of carbon-supported Pt-Bi and Pt-Sb catalysts for the oxidative coupling of 2-Methyl-1-naphthol. Reac Kinet Mech Cat 134, 95–107 (2021). https://doi.org/10.1007/s11144-021-02038-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02038-0