Abstract
An attempt was made to answer the question if spontaneous oscillatory conversion and peptidization of proteinogenic α-amino acids might be essential for living organisms. To this effect, we investigated an impact of heavy water (D2O) on the peptidization of l-Cys. As analytical techniques, we used high-performance liquid chromatography, mass spectrometry, scanning electron microscopy, and turbidimetry. The results obtained demonstrate that heavy water seriously hampers the oscillatory peptidization of l-Cys, apparently due to the presence of the deuterium cation in the reaction medium. A cautious conclusion can be drawn that thorough reflection is needed on possible importance of the oscillatory peptidization of proteinogenic α-amino acids for various different life processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In our studies on the oscillatory chemical reactions initiated in 2005 with paper [1], we have abundantly reported on the phenomena of spontaneous oscillatory chiral inversion and spontaneous oscillatory condensation with the low-molecular-weight carboxylic acids such, as profen drugs, hydroxy acids and proteinogenic α-amino acids (e.g., [1,2,3]).
Among the compounds investigated so far, proteinogenic α-amino acids seem the most significant group, due to their prominent role played in all living organisms. In the experiments demonstrating the phenomena of spontaneous oscillatory chiral inversion and peptidization, many analytical techniques have been engaged such, as polarimetry [1, 4], turbidimetry [5], IR spectroscopy [6], 1H and 13C NMR spectroscopy [7, 8], mass spectrometry [9,10,11] and scanning electron microscopy (SEM) [12], yet the most important techniques were the thin-layer chromatography (TLC) [1] and high-performance liquid chromatography (HPLC) [3]. Moreover, theoretical models were presented in a series of papers [2, 9,10,11, 13,14,15], devised based on general physicochemical knowledge and semi-quantitative assumptions regarding the observed inversion and condensation phenomena, with an aim to add to them a justifiable rationale. Upon an example of l-cysteine, schematic presentation is provided of the processes of chiral inversion and peptidization with proteinogenic α-amino acids and a scheme of these two processes running in the parallel (Fig. S1a–S1c; Supplementary material). It is noteworthy that all these elementary steps are largely based on transfer of the hydrogen cation.
We assume that spontaneous oscillatory peptidization of proteinogenic α-amino acids can take place not only in the test tubes, but in living organisms as well and be responsible for various physiological processes on molecular level. Water is a natural environment for all these processes as the main component of living beings, able to facilitate the mechanisms of hydrogen cation transfer. To get a deeper insight in the processes running in living organisms, the scientists have long explored the role of heavy water (D2O) on the metabolism of many organisms [16,17,18,19,20,21,22,23,24,25,26,27,28,29]. The simplest organisms (such as bacteria, protozoa and algae) have proved to be the most resistant to the toxic effects of heavy water at an expense of usually a not very significant slowdown of their living processes (which is, however, reversible upon bringing back these organisms to H2O). It was demonstrated upon an example of Escherichia coli that this bacterium was able to adapt to and survive in pure heavy water [23]. The organisms with a slightly higher level of cellular organization can also survive either in pure heavy water (algae) [17, 18], or in water considerably enriched with D2O (protozoa) [18]. The toxic influence of heavy water is far more acute with higher organisms such as, e.g., fishes, birds and mammals [18,19,20,21,22]. With certain mammals, lethal effect has been established at the 30% D2O level per the organism’s weight [20]. Besides, on the cellular level it has been proved that D2O strongly affects the processes of mitosis, changes molecular properties of desoxyribonucleic acid, affects separation of the DNA strands and also the course of their further replications [24]. These results gave rise to the expectations on a possibility of including heavy water in the anti-cancer therapies as an apoptotic agent [24,25,26,27,28,29].
A separate and very interesting field of research on the impact induced by D2O on the mechanism and kinetics of the “classical” oscillatory chemical reactions (i.e., the Belousov-Zhabotinsky and Bray-Liebhafsky reaction) has been developed in the span of almost four decades now and the results were presented in a selection of papers (e.g., [30,31,32,33,34,35]). In view of the fact that analytical tools sensitive enough to adequately scrutinize the oscillatory chemical reactions are still rather limited, the insights gained and conclusions derived by individual research groups can only be regarded as fragmentary and hence, as preliminary and tentative only. Nevertheless, each research group has reported an evident impact of D2O on the oscillation dynamics of the processes of interest and some attempts were made to explain the role of heavy water for the selected elementary steps of these processes.
Data on the impact of heavy water on living organisms available in the literature instigated our interest in an effect of D2O on spontaneous peptidization of the proteinogenic α-amino acids. We assumed that the kinetics of the elementary steps of the chiral inversion and peptidization (Fig. S1a–S1c); Supplementary material) might be affected by replacement of hydrogen by deuterium in the reaction environment, leading to perceptible changes in the peptidization dynamics also. For the experiment, we selected l-cysteine (l-Cys) as an important building block of the mammalian proteins and we employed high-performance liquid chromatography with evaporative light-scattering detection (HPLC-ELSD), mass spectrometry (MS), scanning electron microscopy (SEM) and turbidimetry as the measuring techniques.
Experimental
Reagents and samples
l-Cys was of analytical purity, purchased from Reanal (Budapest, Hungary). Heavy water (D2O) was acquired from the Cambridge Isotopic Laboratories (Andover, MA, USA; 99% purity). Water (H2O) was deionized and double distilled by means of an Elix Advantage Millipore system. Acetonitrile (ACN) was of HPLC purity (J.T. Baker, Deventer, the Netherlands).
The l-Cys sample prepared for the HPLC-ELSD experiment was dissolved at a concentration of 0.7 mg mL−1 (5.77 × 10−3 mol L−1) in ACN + H2O, 70:30 (v/v) and the chromatographic measurements of the concentration changes of the monomeric l-Cys were carried out for 95 h at 21 ± 0.5 °C, at the 10-min intervals. The analogous measurements of the concentration changes were carried out for the monomeric l-Cys dissolved in pure D2O.
All the l-Cys solutions used for mass spectrometry, scanning electron microscopy and turbidimetry were prepared at a concentration of 1 mg mL−1 either in pure D2O, or in the binary liquid mixture ACN + X, 70:30 (v/v), where X: the binary mixture of H2O + D2O in the changing volume proportions: 30:0, 29:1, 27:3, 26:4, 25:5, 20:10, 10:20, 5:25, and 0:30.
High-performance liquid chromatography with evaporative light scattering detection (HPLC-ELSD)
High-performance liquid chromatography with evaporative light scattering detection (HPLC-ELSD) was employed to separate the monomeric l-Cys from peptides. The analyses were carried out using a Varian model 920 liquid chromatograph equipped with a 900-LC autosampler, gradient pump, 380-LC ELSD detector and ThermoQuest Hypersil C18 column (150 × 4.6 mm i.d.; 5 μm particle size) for l-Cys and Galaxie software for data acquisition and processing. The chromatographic column was thermostatted at 35 °C using a Varian Pro Star 510 column oven. The chromatographic analyses were carried out using the 10-μL sample aliquots and a methanol–water (80:20, v/v) mobile phase at a flow rate of 0.80 mL min−1. Relatively short sampling time intervals were chosen in order to derive quasi-kinetic information about the oscillatory peptidization.
Mass spectrometry (MS)
All mass spectra were recorded in the positive ionization mode on a Varian MS-100 mass spectrometer (extended ESI–MS scan, positive ionization, spray chamber temperature 50 °C, drying gas temperature 250 °C, drying gas pressure 25 psi, capillary voltage 50 V, needle voltage 5 kV). The mass spectra were recorded for the soluble peptide fraction (as the insoluble microparticle suspensions self-separated by sedimentation) of the ten investigated l-Cys samples immediately after 7 days storage in the measuring cell of turbidimeter.
Scanning electron microscopy (SEM)
Visualization of nano- and microparticles for ten investigated l-Cys samples was performed after 1 month sample storage period with use of a JEOL JSM-7600F model scanning electron microscope (SEM). Visualization was performed for nano and microparticles obtained from the solutions evaporated to dryness.
Turbidimetry
Turbidity measurements were performed for ten investigated l-Cys samples. For this purpose, the turbidity sensor (TRB-BTA, Vernier Software & Technology, Beaverton, OR, USA) was used that allowed continuous monitoring of turbidity changes. For these experiments, ca. 15-mL aliquots of the l-Cys solutions in the solvents were freshly prepared and placed in the instrument cells. The turbidity changes were registered for the period of 7 days (in the 1-min intervals) under the thermostatic conditions at 25.0 ± 0.5 °C. To confirm qualitative reproducibility of the results, the turbidity measurements were repeated twice.
The stability of turbidimeter was controlled for D2O, H2O, ACN, and 70% aqueous ACN as the references (and established as equal to 91.8, 0.0, 80.1 and 40.1 NTU (nephelometric turbidity units) in the course of 20 h. In each case, the turbidity was quite stable (as confirmed by insignificant RSD values).
Results and discussion
High-performance liquid chromatography with evaporative light scattering detection (HPLC-ELSD)
Prior to considering the impact of D2O on the process of spontaneous peptidization of l-Cys, we investigated its behavior when stored for the period from 25 h to 95 h in ACN + H2O (70:30, v/v). In that way, we verified our basic assumption regarding spontaneous oscillatory condensation taking place with chiral low molecular weight carboxylic acids (confirmed with a number of other analytes in our earlier studies, e.g., in [2, 9,10,11, 13,14,15]). Although we are well aware of the fact that condensation of l-Cys consists not only of peptidization, but also of bridging the molecules through the disulfide bonds (as shown in our earlier paper [36]), for the sake of convenience we are going to refer to the condensation as peptidization (keeping in mind that formation of disulfides plays a secondary role in the discussed process).
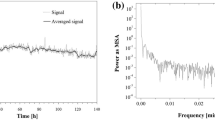
Thus, the achiral HPLC mode was employed to separate the monomeric l-Cys from the spontaneously formed peptides and to check from its changing amount, whether it was undergoing an oscillatory peptidization/hydrolytic de-peptidization process, or not. The chromatogram remained qualitatively unchanged throughout the whole sample storage time (the recorded retention time, tR, was ca. 4.10 min), although quantitative changes of the l-Cys amount were far above an otherwise negligible experimental noise, inherent of the ELSD-type detector. In order to visualize time evolution of the solution, we plotted the changing l-Cys peak heights against the sample storage time (Fig. S2; Supplmentary material). Thus, we saw the non-linear signal intensity changes, which are equivalent to the l-Cys amount changes. Then we Fourier transformed the chromatographic time series in order to check if the HPLC signal for the monomeric l-Cys contains a significant periodic component. The power spectrum calculated for the l-Cys peak is also given in Supplementary material (Fig. S3). It contains a large peak at zero frequency, which was neglected. Then another peak (slightly above the background noise) appears at 0.0007 min−1, implying a periodicity of ca. 24 h. However, the total length of the data is limited to 70 h by our experimental stability and certainly a longer time series would be desirable to confirm this periodicity.
The analogous experiment of storing l-Cys in pure D2O for the period of 72 h resulted in a practically unchanged chromatographic peak height of this compound, equivalent to an unchanged l-Cys amount throughout the whole storage period. Hence, a conclusion was drawn that the environment of heavy water—unlike that consisting of ACN + H2O (70:30, v/v)—fully hampers the process of spontaneous oscillatory peptidization of l-Cys.
Mass spectrometric (MS) tracing of spontaneous peptidization of l-Cys
According to the assumed working procedure, one sample of l-Cys was dissolved in 100% D2O and nine samples of l-Cys were dissolved in the ACN + X, 70:30 (v/v) liquid mixtures, were X: H2O + D2O in different volume proportions (see “Reagents and samples”). The highest volume amount of D2O in the ACN + X mixture was fixed at 30%, based on the Ref. [20] pointing out to this value per organism weight as a lethal amount for experimental mammals. The process of peptidization of each l-Cys sample was running spontaneously at 25 ± 0.5 °C for 7 days in the darkness. After that time, for each sample the mass spectrum was recorded to reveal peptides formed in the course of sample storage. For technical reasons, these mass spectra could be recorded for the monomeric l-Cys and the soluble peptides only, as the insoluble higher peptides self-separate from the solution by sedimentation.
The obtained mass spectra were treated as fingerprints and attentively compared. The mass spectrum recorded for the sample dissolved in pure D2O showed slight contamination of the commercial l-Cys sample with some peptides originating from the manufacturing process and impossible to hydrolyze in D2O (as normally is the case in H2O). For this reason, the mass spectrum recorded for l-Cys dissolved in pure D2O was excluded from further considerations. With the nine l-Cys samples dissolved in ACN + X, the following regularity was observed. For the sample with the volume amount of H2O fixed at 30% (i.e., the volume amount of D2O equal 0%) and for those with relatively low amounts (up to 3%) of D2O in the solvent, the mass spectra showed relatively low numbers of relatively low intensity signals, mostly in the m/z range up to 1000. With the increasing amounts of D2O, the nature of the obtained fingerprints was changing, i.e., more signals started appearing in the whole recorded m/z range (extending up to m/z 3500) and their intensities were considerably higher. Selected examples collected in Fig. 1 well illustrate this tendency.
At a first glance, the mass spectra obtained in our experiment seem witnessing to the fact that it is H2O which hampers peptidization and D2O which stimulates it. However, such conclusion is not correct, if we keep in mind that the mass spectrometric results are obtained for soluble (hence, the lower molecular weight) peptides only. The complementary results originating from the scanning electron microscopy (SEM) (discussed in the next section) witness to the opposite. The SEM results presenting peptide nano- and microstructures cumulated in the considered solutions clearly prove that the more H2O is in a solvent, the bigger are the obtained peptide structures (and consequently the less soluble as well). In other words, H2O pushes an overall equilibrium of peptidization toward the higher (largely insoluble) peptides, whereas peptidization in the presence of D2O obstructs it, leading toward the lower (and mostly soluble) peptides.
Scanning electron microscopic (SEM) tracing of spontaneous peptidization of l-Cys
With the mass spectra recorded for the samples with increasing quantitative proportions of H2O in solution, the general trend of the lowering yields of the soluble l-Cys-derived peptides was observed (Fig. 1). On the other hand, from our earlier studies on peptidization of l-Cys in ACN + H2O (70:30, v/v), it clearly came out that its spontaneous peptidization was fast and within a few days of sample storage insoluble peptides abundantly floating in solution were easily perceptible with naked eye (see Fig. 2 in [30]). Thus a conclusion was drawn that the mass spectrometric evidence of the diminishing yields of soluble peptides with the increasing quantitative proportions of H2O did not reflect the overall peptidization yields, as the higher insoluble peptides escaped the mass spectrometric evidence. To this effect, we compared average yields and sizes of insoluble l-Cys-derived peptides in ten investigated solutions with use of the scanning electron microscopy (SEM). Selected micrographs which well illustrate the observed regularities and trend are given in Fig. 2.
Scanning electron micrographs recorded for the l-Cys-derived peptides retrieved from the samples dissolved in ACN + X, 70:30 (v/v). X: the binary mixture of H2O + D2O in the changing volume proportions; a 0% D2O, × 100,000; b 3% D2O, × 50,000; c 10% D2O, × 100,000; d 20% D2O, × 30,000; e 30% D2O, × 35,000; f 100% D2O, × 37,000
Micrographs of the higher and mostly insoluble l-Cys-derived peptides show lowering of peptide yields with the increasing amounts of D2O in the solvent (Fig. 2). Peptides formed in an absence of D2O (Fig. 2a) and in the presence of 3 and 10% D2O (Fig. 2b, c) show a compact peptide matter formed of stuck together globular particles up to ca. 100 nm diameter, just on the borderline between nano- and microparticles. The texture of the peptide matter formed in the presence of 20% D2O is perceptibly less compact (Fig. 2d) and aggregations in solution containing 30% D2O peptide are even smaller (Fig. 2e). The micrograph valid for l-Cys stored in pure D2O shows the smallest peptide aggregations of them all (Fig. 2f). In each case, average diameters of single globular particles were comparable (in the range of up to ca. 100 nm) and hence, it can be concluded that the increasing amounts of D2O result in lowering of peptide yields viewed as the less numerous aggregations, but not necessarily in lowering of the average particle sizes.
Turbidimetric tracing of spontaneous peptidization of l-Cys
In view of a lack of standardization in turbidity units, measurement devices and calibration techniques, usage of turbidimetry to analytical determinations can only be empirical and rather qualitative [37]. However, from our earlier turbidimetric investigations it comes out that even, if—due to certain randomness of spontaneous peptidization—the absolute turbidity values can slightly differ from one experiment to the other, the patterns remain very similar in each repetition [38]. The plots of turbidity changes were recorded in the 1-min intervals for the period of 7 days for one l-Cys sample dissolved in pure D2O and nine l-Cys samples dissolved in the ACN + X, 70:30 (v/v) mixtures. They revealed differences in patterns and hence, in peptidization dynamics visibly correlated with an amount of D2O in a given system. Selected results well illustrating the trend of the observed changes are shown in Fig. 3.
Turbidity changes (in nephelometric turbidity units, NTU) in the period between 0 and 7 days measurement for the l-Cys solution in ACN + X, 70:30 (v/v). X: the binary mixture of H2O + D2O with the changing volume proportions: a 1% D2O, b 3% D2O, and c 20% D2O. As a reference, d pure D2O is considered. Arrows indicate the end of the initial stagnation of the turbidity plot
In each turbidity plot shown in Fig. 3, an initial sharp signal drift lasting a few hours was observed, due to adjusting the sample’s temperature to 25.0 ± 0.5 °C (as turbidity strongly depends on density of liquid sample and prior to the experiment, D2O was kept in refrigerator). Let us start our comparison from Fig. 3d valid for pure D2O as a reference. After an initial sharp signal drop, for the rest of the 7 days sample storage, a fairly stagnant plot was observed with not very prominent turbidity changes. To the contrary, the turbidity plot for l-Cys with 1% D2O evidently became dynamic with turbidity values non-monotonously changing after the first day of relative stagnation (as indicated with an arrow; Fig. 3a). This plot is almost identical to that valid for l-Cys in complete absence of D2O. In Fig. 3b, we show the turbidity plot for l-Cys with 3% D2O and although the growth of D2O concentration is relatively small, it is reflected in perceptible prolongation of preliminary stagnation to 2 days. Only then, certain dynamics of the turbidity pattern (and more precisely, the stepwise turbidity drop) was observed. Addition of 20% D2O resulted in prolongation of the initial stagnation period to over 3 days and only then, the gradual and not very strongly pronounced turbidity drop began. In conclusion, it can be stated that the growing amount of D2O in solution was perceptibly changing the l-Cys turbidity pattern, making it increasingly more stagnant.
Summing up, from the experimental results originating from several analytical techniques presented in this study, a conclusion could be drawn that spontaneous oscillatory peptidization of l-Cys is hampered by the presence of D2O in liquid systems. This hampering effect could be twofold. First, one can expect purely physical interactions of D2O with the l-Cys molecules like the dipole–dipole interactions, formation of H-bonds etc., all of them able to affect the rate constants of the elementary processes shown in Supplementary material. Secondly, the impact of D2O could also be of a chemical nature, through the isotopic exchange of the l-Cys protons for the deuterons. With the concentration excess of D2O over l-Cys, such an isotopic exchange can occur on each hydrogen atom in the l-Cys molecule, although the energetically and mechanistically most meaningful exchange might be expected on the –COOH, –NH2 and –SH functionalities, turning them to –COOD, –NHD, –ND2 and –SD. Detailed reflections on the contributions of D2O to hampering spontaneous oscillatory peptidization will be the subject matter of our future studies.
In a general sense, the experimental results obtained in this study remain in agreement with those provided in the earlier papers on the impact of D2O on the known oscillatory processes [30,31,32,33,34,35]. The results presented in papers [32, 33] emphasize the change of the reaction mechanism (i.e., the kinetic pathways) of the Bray-Liebhafsky reaction under the influence of heavy water. In paper [35], the impact of heavy water on the kinetics of the Belousov-Zhabotinsky reaction was demonstrated and more specifically, an evident slowdown of this reaction due to considerable prolongation of its induction period. In our study, the impact of heavy water expressed itself by a practical standstill of the oscillatory process of peptidization.
Conclusion
In this study, it was once again demonstrated that spontaneous peptidization of l-Cys is an oscillatory process. Hampering oscillations of l-Cys with aid of D2O was investigated for the first time and it proves practically unequivocal with hampering peptidization. Now a future and thorough reflection is needed on the importance of the oscillatory peptidization of the proteinogenic α-amino acids for various different life processes, the phenomenon which might carry some evolutionary implications.
References
Sajewicz M, Piętka R, Pieniak A, Kowalska T (2005) Application of thin-layer chromatography (TLC) to investigating oscillatory instability of the selected profen enantiomers. Acta Chromatogr 15:131–149
Sajewicz M, Gontarska M, Wojtal Ł, Kronenbach D, Leda M, Epstein IR, Kowalska T (2008) Experimental and model investigation of the oscillatory transenantiomerization of l-α-phenylalanine. J Liq Chromatogr Relat Technol 31:1986–2005
Sajewicz M, Gontarska M, Kowalska T (2014) HPLC/DAD evidence of the oscillatory chiral conversion of phenylglycine. J Chromatogr Sci 52:329–333
Sajewicz M, Piętka R, Pieniak A, Kowalska T (2005) Application of thin-layer chromatography (TLC) to investigate oscillatory instability of the selected profen enantiomers in dichloromethane. J Chromatogr Sci 43:542–548
Maciejowska A, Godziek A, Sajewicz M, Kowalska T (2017) Turbidity patterns of spontaneous peptidization in an aqueous abiotic system and possible secondary peptide structures. Reac Kinet Mech Cat 120:421–437
Godziek A, Maciejowska A, Talik E, Wrzalik R, Sajewicz M, Kowalska T (2016) On spontaneously pulsating proline-phenylalanine peptide microfibers. Curr Prot Pept Sci 17:106–116
Matlengiewicz M, Sajewicz M, Gontarska M, Kronenbach D, Kowalska T (2010) On the spontaneous condensation of profens, with ketoprofen as an example. Acta Chromatogr 22:81–90
Sajewicz M, Matlengiewicz M, Juziuk M, Penkala M, Weloe M, Schulz M, Kowalska T (2013) Thin-layer chromatographic evidence of proline peptidization in solution and its thin-layer chromatographic enantioseparation. J Liq Chromatogr Relat Technol 36:2497–2511
Sajewicz M, Gontarska M, Kronenbach D, Leda M, Kowalska T, Epstein IR (2010) Condensation oscillations in the peptidization of phenylglycine. J Syst Chem. https://doi.org/10.1186/1759-2208-1-7
Sajewicz M, Matlengiewicz M, Leda M, Gontarska M, Kronenbach D, Kowalska T, Epstein IR (2010) Spontaneous oscillatory in vitro chiral conversion of simple carboxylic acids and its possible mechanism. J Phys Org Chem 23:1066–1073
Sajewicz M, Dolnik M, Kowalska T, Epstein IR (2014) Condensation dynamics of l-proline and l-hydroxyproline in solution. RSC Adv 4:7330–7339
Godziek A, Maciejowska A, Talik E, Sajewicz M, Kowalska T (2016) Scanning electron microscopic evidence of spontaneous heteropeptide formation in abiotic solutions of selected α-amino acid pairs. Isr J Chem 56:1057–1066
Sajewicz M, Wrzalik R, Gontarska M, Kronenbach D, Leda M, Epstein IR, Kowalska T (2009) In vitro chiral conversion, phase separation, and wave propagation in aged profen solutions. J Liq Chromatogr Relat Technol 32:1359–1372
Sajewicz M, Dolnik M, Kronenbach D, Gontarska M, Kowalska T, Epstein IR (2011) Oligomerization oscillations of l-lactic acid in solutions. J Phys Chem A 115:14331–14339
Maciejowska A, Godziek A, Talik E, Sajewicz M, Kowalska T, Epstein IR (2016) Spontaneous pulsation of peptide microstructures in an abiotic liquid system. J Chromatogr Sci 54:1301–1309
De Giovanni R (1961) The effect of deuterium oxide on bacteria. Z Vererbungsl 92:389–402
Daboll HF, Crespi HL, Katz JJ (1962) Mass cultivation of algae in pure heavy water. Biotechnol Bioeng 4:281–297
Katz JJ, Crespi HL, Czajka DM, Finkel AJ (1962) Course of deuteriation and some physiological effects of deuterium in mice. Am J Physiol 203:907–913
Bachner P, McKay DG, Rittenberg D (1964) The pathological anatomy of deuterium intoxication. Proc Natl Acad Sci USA 51:464–471
Richter CP (1976) A study of taste and smell of heavy water (98%) in rats. Proc Soc Exp Biol Med 152:677–684
Richter CP (1977) Heavy water as a tool for study of the forces that control length of period of the 24-hour clock of the hamster. Proc Natl Acad Sci USA 74:1295–1299
Kanto U, Clawson AJ (1980) Use of deuterium oxide for the in vivo prediction of body composition in female rats in various physiological states. J Nutrit 110:1840–1848
Litvinenko LA, Kravchuk LA, Petrikevich SB, Sakharovskii VG, Ivanitskaia JG, Guliamova DE (1992) Effect of heavy water on the growth, glucose assimilation and stability of Escherichia coli to freezing-thawing. Mikrobiologiia 61:1030–1037 (in Russian)
Schroeter D, Lamprecht J, Eckhardt R, Futterman G, Paweletz N (1992) Deuterium oxide (heavy water) arrests the cell cycle of PtK2 cells during interphase. Eur J Cell Biol 58:365–370
Takeda H, Nio Y, Omori H, Uegaki K, Hirahara N, Sasaki S, Tamura K, Ohtani H (1998) Mechanisms of cytotoxic effects of heavy water (deuterium oxide: D2O) on cancer cells. Anticancer Drugs 9:715–725
Kushner DJ, Baker A, Dunstall TG (1999) Pharmacological uses and perspectives of heavy water and deuterated compounds. Can J Physiol Pharmacol 77:79–88
Busch R, Kim Y, Neese RA, Schade-Serin V, Collins M, Awada M, Gardner JL, Beysen C, Marino ME, Misell LM, Hellerstein MK (2006) Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta 1760:730–744
Demidov VV (2007) Heavy isotopes to avert ageing? Trends Biotechnol 25:371–375
Kumar N, Attri P, Kumar Yadav D, Choi J, Choi EH, Uhm HS (2014) Induced apoptosis in melanocytes cancer cell and oxidation in biomolecules through deuterium oxide generated from atmospheric pressure non-thermal plasma jet. Sci Rep. https://doi.org/10.1038/srep07589
Kreuels T, Martin W, Brinkmann K (1980) Influence of D2O on the Belousov-Zhabotinsky Reaction. Ber Bunsenges Phys Chem 84:411–412
Karavaev AD, Kazakov VP, Tolstikov GA (1986) Deuteration effect in auto-oscillation chemiluminescence of the Belousov-Zhabotinskii reaction. Reac Kinet Catal Lett 32:21–26
Stanisavljev DR, Vukojević VB (1998) Investigation of the influence of heavy water on kinetic pathways in the Bray-Leiebhafsky reaction. J Phys Chem A 102:5618–5625
Stanisavljev D, Begović N, Vukojević V (1998) Influence of heavy water in the Bray-Liebhafsky oscillating reaction. J Phys Chem A 102:6887–6891
Hsu M-C, Jwo J-J (1999) Kinetic study of the isotope exchange reactions of malonic acid and its derivatives in various acidic D2O media using 1H NMR spectroscopy. Implication in the Belousov-Zabotinsky reaction. Int J Chem Kinet 31:455–461
Rossi F, Rustic M, Rossi C, Tiezzi E (2007) Isotopic effect on the kinetics of the Belousov-Zhabotinsky reaction. Int J Mol Sci 8:943–949
Godziek A, Maciejowska A, Talik E, Sajewicz M, Kowalska T (2015) Thin-layer investigation of l-cysteine in solution. J Planar Chromatogr 28:144–151
Lawler DM (2005) Turbidity and nephelometry. In: Worsfold P, Townshend A, Poole C (eds) Encyclopedia of analytical science, 2nd edn. Elsevier Academic, San Diego, pp 343–352
Maciejowska A, Godziek A, Sajewicz M, Kowalska T (2017) Turbidity patterns of spontaneous peptidization in an aqueous abiotic system and possible secondary peptide structures. Reac Kinet Mech Cat 120:421–437
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fulczyk, A., Łata, E., Dolnik, M. et al. Impact of D2O on peptidization of l-Cysteine. Reac Kinet Mech Cat 125, 555–565 (2018). https://doi.org/10.1007/s11144-018-1469-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-018-1469-y