Abstract
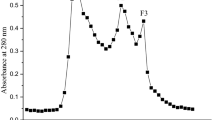
To obtain Angiotensin-I-Converting Enzyme (ACE) inhibition peptides with Zn-chelating capacity, quinoa bran glutelin-2 hydrolysates (QBGH) by Flavourzyme and Papain were subjected to Sephadex G-15 gel chromatography, reverse phase-high liquid performance chromatography and UPLC-ESI–MS/MS analysis. Four oligopeptides including GGGSGH, EAGAE, AGGGAGGG and AVPKPS were identified. Of these, only the hexapeptide AVPKPS had both ACE-inhibitory activity (IC50: 123.13 μmol/L) and Zn-chelating ability (17.36 mg/g). Molecular docking showed AVPKPS could bind with active residues Glu384 and Ala354 (both belong to the central S1 pocket of ACE including) through short hydrogen bond and hydrophobic interactions, respectively. Inhibition kinetics verified that AVPKPS was a competitive inhibitor of ACE. Moreover, AVPKPS can affect the zinc tetrahedral coordination in ACE through binding with residues His387 and His383. Fourier-transform infrared spectroscopy analysis demonstrated that the amino and carboxyl groups of AVPKPS were the main chelating sites for zinc ions. Under the gastrointestinal digestion, the ACE inhibition capacity of AVPKPS was relatively stable, and the zinc solubility of AVPKPS-zinc complexes was more stable than zinc sulfate (p < 0.05). These results suggest that quinoa peptides have potential applications as ingredients for antihypertension or zinc fortification.







Similar content being viewed by others
Data Availability
Data will be availability on a reasonable requirement.
References
Fan H, Wu J (2021) Purification and identification of novel ACE inhibitory and ACE2 upregulating peptides from spent hen muscle proteins. Food Chem 398:957–980
Li R, Zhou X, Sun L, Zhuang Y (2022) Identification, in silico screening, and molecular docking of novel ACE inhibitory peptides isolated from the edible symbiot Boletus griseus-Hypomyces chrysospermus. LWT-Food Sci Technol 169:114008
Lee SY, Hur SJ (2017) Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem 228:506–517
Sun R, Liu X, Yu Y, Miao J, Leng K, Gao H (2021) Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides-zinc chelate. Food Chem 340:128056
Ke X, Hu X, Li L, Yang X, Chen S, Wu Y, Xue C (2022) A novel zinc-binding peptide identified from tilapia (Oreochromis niloticus) skin collagen and transport pathway across Caco-2 monolayers. Food Biosci 42:101127
Wang K, Luo Q, Hong H, Liu H, Luo Y (2021) Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res Inter 139:109908
González-Muñoz A, Valle M, Aluko RE, Bazinet L, Enrione J (2022) Production of antihypertensive and antidiabetic peptide fractions from quinoa (Chenopodium quinoa Willd.) by electrodialysis with ultrafi ltration membranes. Food Sci Hum Wellness 11:1650–1659
Dakhili S, Abdolalizadeh L, Hosseini SM, Shojaee-Aliabadi S, Mirmoghtadaie L (2019) Quinoa protein: Composition, structure and functional properties. Food Chem 299:125161
Ujiroghene J, Liu L, Zhang S, Lu J, Pang XY, Lv JP (2019) α-Glucosidase and ACE dual inhibitory protein hydrolysates and peptide fractions of sprouted quinoa yoghurt beverages inoculated with Lactobacillus casei. Food Chem 299:124985
Wei GQ, Zhao Q, Wang DD, Fan YZ, Shi YN, Huang AX (2022) Novel ACE inhibitory, antioxidant and α-glucosidase inhibitory peptides identified from fermented rubing cheese through peptidomic and molecular docking. LWT-Food Sci Technol 159:113196
Chen J, Yu X, Chen Q, Wu Q, He Q (2022) Screening and mechanisms of novel angiotensin-I-converting enzyme inhibitory peptides from rabbit meat proteins: A combined in silico and in vitro study. Food Chem 370:131070
Zaharuddin ND, Barkia I, Ibadullah WZW, Zarei NS (2022) Identification, molecular docking, and kinetic studies of six novel angiotensin-I-converting enzyme (ACE) inhibitory peptides derived from Kenaf (Hibiscus cannabinus L.) seed. Int J Biol Macromol 220:1512–1522
Zhu S, Zheng Y, He S, Su D, Nag A, Zeng Q, Yuan Y (2021) Novel Zn-Binding Peptide Isolated from Soy Protein Hydrolysates: Purification, Structure, and Digestion. J Agri Food Chem 69(1):483–490
Hou H, Wang S, Zhu X, Li Q, Fan Y, Cheng D, Li B (2018) A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex. Food Chem 243:389–395
Zarei M, Ghanbari R, Zainal N, Ovissipour R, Saari N (2022) Inhibition kinetics, molecular docking, and stability studies of the effect of papain-generated peptides from palm kernel cake proteins on angiotensin-converting enzyme (ACE). Food Chem Mol Sci 5:100147
Cao X, Yang J, Ma H, Guo P, Cai Y, Xu H, Ding G, Gao D (2021) Angiotensin I Converting Enzyme (ACE) inhibitory peptides derived from alfalfa (Medicago sativa L.) leaf protein and its membrane fractions [J]. J Food Process Pres 45:10
Funding
This work was supported by the Natural Science Foundation of Shanxi Province, China (202203021221139).
Author information
Authors and Affiliations
Contributions
Junru Li: Investigation, Methodology. Xinyu Huo: Investigation, Writing-review. Yajun Zheng: Conceptualization, Writing-original draft, Funding acquisition. Yizi Guo: Validation. Chen Feng: Writing-review.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Huo, X., Zheng, Y. et al. ACE-Inhibitory Peptides Identified from Quinoa Bran Glutelin-2 Hydrolysates: In Silico Screening and Characterization, Inhibition Mechanisms of ACE, Coordination with Zinc Ions, and Stability. Plant Foods Hum Nutr 78, 419–425 (2023). https://doi.org/10.1007/s11130-023-01074-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-023-01074-6