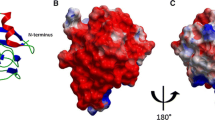
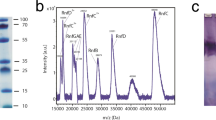
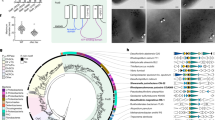
Abstract
The homodimeric Type I reaction center (RC) from Heliomicrobium modesticaldum lacks the PsaC subunit found in Photosystem I and instead uses the interpolypeptide [4Fe–4S] cluster FX as the terminal electron acceptor. Our goal was to identify which of the small mobile dicluster ferredoxins encoded by the H. modesticaldum genome are capable of accepting electrons from the heliobacterial RC (HbRC) and pyruvate:ferredoxin oxidoreductase (PFOR), a key metabolic enzyme. Analysis of the genome revealed seven candidates: HM1_1462 (PshB1), HM1_1461 (PshB2), HM1_2505 (Fdx3), HM1_0869 (FdxB), HM1_1043, HM1_0357, and HM1_2767. Heterologous expression in Escherichia coli and studies using time-resolved optical spectroscopy revealed that only PshB1, PshB2, and Fdx3 are capable of accepting electrons from the HbRC and PFOR. Modeling studies using AlphaFold show that only PshB1, PshB2, and Fdx3 should be capable of docking on PFOR at a positively charged patch that overlays a surface-proximal [4Fe–4S] cluster. Proteomic analysis of wild-type and gene deletion strains ΔpshB1, ΔpshB2, ΔpshB1pshB2, and Δfdx3 grown under nitrogen-replete conditions revealed that Fdx3 is undetectable in the wild-type, ΔpshB1, and Δfdx3 strains, but it is present in the ΔpshB2 and ΔpshB1pshB2 strains, implying that Fdx3 may substitute for PshB2. When grown under nitrogen-deplete conditions, Fdx3 is present in the wild-type and all deletion strains except for Δfdx3. None of the knockout strains demonstrated significant impairment during chemotrophic dark growth on pyruvate, photoheterotrophic light growth on pyruvate, or phototrophic growth on acetate+CO2, indicating a high degree of redundancy among these three electron transfer proteins. Loss of both PshB1 and PshB2, but not FdxB, resulted in poor growth under N2-fixing conditions.










Similar content being viewed by others
References
Antonkine ML, Golbeck JH (2006) Molecular interactions of the stromal subunit PsaC with the PsaA/PsaB heterodimer. In: Golbeck JH (ed) Photosystem I: the light-driven plastocyanin: ferredoxin oxidoreductase. Springer, Dordrecht, pp 79–98
Asao M, Madigan MT (2010) Taxonomy, phylogeny, and ecology of the heliobacteria. Photosynth Res 104(2–3):103–111
Baker PL, Orf GS, Khan Z, Espinoza L, Leung S, Kevershan K, Redding KE (2019) A molecular biology tool kit for the phototrophic firmicute Heliobacterium modesticaldum. Appl Environ Microbiol 85(19):e01287–e1319
Baur JR, Graves MC, Feinberg BA, Ragsdale SW (1990) Characterization of the recombinant Clostridium pasteurianum ferredoxin and comparison of its properties with those of the native protein. BioFactors (Oxford, England) 2(3):197–203
Beinert H (1983) Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron-sulfur proteins. Anal Biochem 131(2):373–378
Bern M, Kil YJ, Becker C (2012) Byonic: advanced peptide and protein identification software. Curr Protoc Bioinformatics 13:13.20.1-13.20.14
Brockmann H, Lipinski A (1983) Bacteriochlorophyll g. A new bacteriochlorophyll from Heliobacterium chlorum. Arch Microbiol 136(1):17–19
Bryantseva IA, Gorlenko VM, Kompantseva EI, Achenbach LA, Madigan MT (1999) Heliorestis daurensis, gen. nov. sp. nov., an alkaliphilic rod-to-coiled-shaped phototrophic heliobacterium from a Siberian soda lake. Arch Microbiol 172(3):167–174
Cardona T (2015) A fresh look at the evolution and diversification of photochemical reaction centers. Photosynth Res 126(1):111–134
Cavazza C, Contreras-Martel C, Pieulle L, Chabrière E, Hatchikian EC, Fontecilla-Camps JC (2006) Flexibility of thiamine diphosphate revealed by kinetic crystallographic studies of the reaction of pyruvate-ferredoxin oxidoreductase with pyruvate. Structure 14(2):217–224
Díaz-Quintana A, Leibl W, Bottin H, Sétif P (1998) Electron transfer in photosystem I reaction centers follows a linear pathway in which iron-sulfur cluster FB is the immediate electron donor to soluble ferredoxin. Biochemistry 37(10):3429–3439
Ferlez B, Cowgill J, Dong W, Gisriel C, Lin S, Flores M, Walters K, Cetnar D, Redding KE, Golbeck JH (2016) Thermodynamics of the electron acceptors in Heliobacterium modesticaldum: an exemplar of an early homodimeric type I photosynthetic reaction center. Biochemistry 55(16):2358–2370
Fixen KR, Pal Chowdhury N, Martinez-Perez M, Poudel S, Boyd ES, Harwood CS (2018) The path of electron transfer to nitrogenase in a phototrophic alpha-proteobacterium. Environ Microbiol 20(7):2500–2508
Gisriel C, Sarrou I, Ferlez B, Golbeck JH, Redding KE, Fromme R (2017) Structure of a symmetric photosynthetic reaction center–photosystem. Science (new York, NY) 357(6355):1021–1025
Goddard TD, Huang CC, Meng EC, Pettersen EF, Couch GS, Morris JH, Ferrin TE (2018) UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci 27(1):14–25
Golbeck JH (1993) Shared thematic elements in photochemical reaction centers. Proc Natl Acad Sci USA 90(5):1642–1646
Golbeck JH, van der Est A (2022) EPR of Type I photosynthetic reaction centers. Methods Enzymol 666:413–450
Gómez-Moreno C, Martínez-Júlvez M, Medina M, Hurley JK, Tollin G (1998) Protein-protein interaction in electron transfer reactions: the ferrodoxin/flavodoxin/ferredoxin:NADP+ reductase system from Anabaena. Biochimie 80(10):837–846
Hatano A, Seo D, Kitashima M, Sakurai H, Inoue K (2005) Purification of two ferredoxins and cloning of these genes from the photosynthetic bacterium Heliobacillus mobilis. In: van der Est A, Bruce D (eds) 13th international congress on photosynthesis. Allen Press Inc., Lawrence, KS, pp 83–85
Heathcote P, Jones MR, Fyfe PK (2003) Type I photosynthetic reaction centres: structure and function. Philos Trans R Soc Lond B Biol Sci 358(1429):231–243
Hippler M, Nelson N (2021) The plasticity of photosystem I. Plant Cell Physiol 62(7):1073–1081
Hong JS, Rabinowitz JC (1970) Molar extinction coefficient and iron and sulfide content of Clostridial ferredoxin. J Biol Chem 245(19):4982–4987
Jagannathan B, Golbeck JH (2008) Unifying principles in homodimeric type I photosynthetic reaction centers: properties of PscB and the FA, FB and FX iron–sulfur clusters in green sulfur bacteria. Biochim Biophys Acta Bioenerg 1777(12):1535–1544
Jagannathan B, Shen G, Golbeck JH (2012) The evolution of Type I reaction centers: the response to oxygenic photosynthesis. In: Burnap R, Vermaas W (eds) Functional genomics and evolution of photosynthetic systems. Springer, Dordrecht, pp 285–316
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596(7873):583–589
Kennedy MC, Kent T, Emptage M, Merkle H, Beinert H, Münck E (1984) Evidence for the formation of a linear [3Fe–4S] cluster in partially unfolded aconitase. J Biol Chem 259(23):14463–14471
Kimble LK, Stevenson AK, Madigan MT (1994) Chemotrophic growth of heliobacteria in darkness. FEMS Microbiol Lett 115(1):51–55
Kimble LK, Mandelco L, Woese CR, Madigan MT (1995) Heliobacterium modesticaldum, sp. nov., a thermophilic heliobacterium of hot springs and volcanic soils. Arch Microbiol 163(4):259–267
Kleinherenbrink FAM, Amesz J (1993) Stoichiometries and rates of electron transfer and charge recombination in Heliobacterium chlorum. Biochim Biophys Acta Bioenerg 1143(1):77–83
Leung SW, Baker PL, Redding KE (2021) Deletion of the cytochrome bc complex from Heliobacterium modesticaldum results in viable but non-phototrophic cells. Photosynth Res 148(3):137–152
Moser CC, Page CC, Cogdell RJ, Barber J, Wraight CA, Dutton PL (2003) Length, time, and energy scales of photosystems. Adv Protein Chem 63:71–109
Nelson N (2013) Evolution of photosystem I and the control of global enthalpy in an oxidizing world. Photosynth Res 116(2–3):145–151
Orf GS, Redding KE (2019) Expression and purification of affinity-tagged variants of the photochemical reaction center from Heliobacterium modesticaldum. Photosynth Res 142(3):335–348
Orf GS, Redding KE (2021) Photosynthesis | The heliobacteria. In: Jez J (ed) Encyclopedia of biological chemistry III (third edition). Elsevier, Oxford, pp 352–364
Orf GS, Gisriel C, Redding KE (2018) Evolution of photosynthetic reaction centers: insights from the structure of the heliobacterial reaction center. Photosynth Res 138(1):11–37
Orf GS, Gisriel CJ, Granstrom J, Baker PL, Redding KE (2022) The PshX subunit of the photochemical reaction center from Heliobacterium modesticaldum acts as a low-energy antenna. Photosynth Res 151(1):11–30
Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, Ferrin TE (2021) UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci 30(1):70–82
Pierella Karlusich JJ, Carrillo N (2017) Evolution of the acceptor side of photosystem I: ferredoxin, flavodoxin, and ferredoxin-NADP+ oxidoreductase. Photosyn Res 134:235–250
Prince RC, Gest H, Blankenship RE (1985) Thermodynamic properties of the photochemical reaction center of Heliobacterium chlorum. Biochim Biophys Acta Bioenerg 810(3):377–384
Richard E, Michael ON, Alexander P, Natasha A, Andrew S, Tim G, Augustin Ž, Russ B, Sam B, Jason Y, Olaf R, Sebastian B, Michal Z, Alex B, Anna P, Andrew C, Kathryn T, Rishub J, Ellen C, Pushmeet K, John J, Demis H (2022) Protein complex prediction with AlphaFold-Multimer. bioRxiv 2021.10.04.463034. https://www.biorxiv.org/content/10.1101/2021.10.04.463034v2
Romberger SP, Golbeck JH (2012) The FX iron-sulfur cluster serves as the terminal bound electron acceptor in heliobacterial reaction centers. Photosynth Res 111(3):285–290
Romberger SP, Castro C, Sun Y, Golbeck JH (2010) Identification and characterization of PshBII, a second FA/FB-containing polypeptide in the photosynthetic reaction center of Heliobacterium modesticaldum. Photosynth Res 104(2):293–303
Rutherford AW, Osyczka A, Rappaport F (2012) Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: redox tuning to survive life in O2. FEBS Lett 586(5):603–616
Sattley WM, Blankenship RE (2010) Insights into heliobacterial photosynthesis and physiology from the genome of Heliobacterium modesticaldum. Photosynth Res 104(2):113–122
Sattley WM, Madigan MT, Swingley WD, Cheung PC, Clocksin KM, Conrad AL, Dejesa LC, Honchak BM, Jung DO, Karbach LE, Kurdoglu A, Lahiri S, Mastrian SD, Page LE, Taylor HL, Wang ZT, Raymond J, Chen M, Blankenship RE, Touchman JW (2008) The genome of Heliobacterium modesticaldum, a phototrophic representative of the Firmicutes containing the simplest photosynthetic apparatus. J Bacteriol 190(13):4687–4696
Sattley WM, Asao M, Tang JK-H, Collins AM (2014) Energy conservation in heliobacteria: photosynthesis and central carbon metabolism. In: Hohmann-Marriott MF (ed) The structural basis of biological energy generation. Springer, Dordrecht, pp 231–247
Sheehy D, Kuang Lu Y, Osman F, Alattar Z, Flores C, Sussman H, Zaare S, Dooling M, Meraban A, Baker P (2018) Genome-wide transcriptional response during the shift to N2-fixing conditions in Heliobacterium modesticaldum. J Proteom Bioinform 11(8):143–160
Shen G, Antonkine ML, van der Est A, Vassiliev IR, Brettel K, Bittl R, Zech SG, Zhao J, Stehlik D, Bryant DA, Golbeck JH (2002) Assembly of Photosystem I: II. Rubredoxin is required for the in vivo sssembly of FX in Synechococcus sp. PCC 7002 as shown by optical and EPR spectroscopy. J Biol Chem 277(23):20355–20366
Sobel BE, Lovenberg W (1966) Characteristics of Clostridium pasteurianum ferredoxin in oxidation-reduction reactions. Biochemistry 5(1):6–13
Srivastava AP, Hardy EP, Allen JP, Vaccaro BJ, Johnson MK, Knaff DB (2017) Identification of the ferredoxin-binding site of a ferredoxin-dependent cyanobacterial nitrate reductase. Biochemistry 56(41):5582–5592
Tang KH, Feng X, Zhuang WQ, Alvarez-Cohen L, Blankenship RE, Tang YJ (2010a) Carbon flow of heliobacteria is related more to clostridia than to the green sulfur bacteria. J Biol Chem 285(45):35104–35112
Tang K-H, Yue H, Blankenship RE (2010b) Energy metabolism of Heliobacterium modesticaldum during phototrophic and chemotrophic growth. BMC Microbiol 10(1):150
Vassiliev IR, Antonkine ML, Golbeck JH (2001) Iron–sulfur clusters in type I reaction centers. Biochim Biophys Acta Bioenerg 1507(1):139–160
Walters KA, Golbeck JH (2020) Designing a modified clostridial 2[4Fe–4S] ferredoxin as a redox coupler to directly link photosystem I with a Pt nanoparticle. Photosynth Res 143(2):165–181
Xu Q, Chitnis PR (1995) Organization of photosystem I polypeptides: identification of PsaB domains that may interact with PsaD. Plant Physiol 108(3):1067–1075
Acknowledgements
We wish to thank Patricia Baker for technical assistance with construction of the gene knockouts and growth studies.
Funding
This work was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0010575.
Author information
Authors and Affiliations
Contributions
JG, KM, and KR wrote the main manuscript text and jointly prepared the figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial interests that are either directly or indirectly related to the work submitted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Walters, K.A., Redding, K.E. & Golbeck, J.H. Identification and characterization of the low molecular mass ferredoxins involved in central metabolism in Heliomicrobium modesticaldum. Photosynth Res (2024). https://doi.org/10.1007/s11120-023-01069-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11120-023-01069-z