Abstract
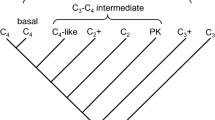
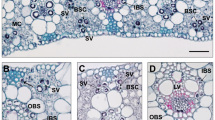
Carbon concentrating mechanisms (CCMs) in plants are abaptive features that have evolved to sustain plant growth in unfavorable environments, especially at low atmospheric carbon levels and high temperatures. Uptake of CO2 and its storage in the aerenchyma tissues of Lycopsids and diurnal acidity fluctuation in aquatic plants during the Palaeozoic era (ca. 300 Ma.) would represent the earliest evolution of a CCM. The CCM parts of the dark reactions of photosynthesis have evolved many times, while the light reactions are conserved across plant lineages. A C4 type CCM, leaf C4 photosynthesis is evolved in the PACMAD clade of the Poaceae family. The evolution of C4 photosynthesis from C3 photosynthesis was an abaptation. Photosynthesis in reproductive tissues of sorghum and maize (PACMAD clade) has been shown to be of a weaker C4 type (high CO2 compensation point, low carbon isotope discrimination, and lack of Rubisco compartmentalization, when compared to the normal C4 types) than that in the leaves (normal C4 type). However, this does not fit well with the character polarity concept from an evolutionary perspective. In a recent model proposed for CCM evolution, the development of a rudimentary CCM prior to the evolution of a more efficient CCM (features contrasting to a weaker C4 type, leading to greater biomass production rate) has been suggested. An intermediate crassulacean acid metabolism (CAM) type of CCM (rudimentary) was reported in the genera, Brassia, Coryanthes, Eriopsis, Peristeria, of the orchids (well-known group of plants that display the CAM pathway). Similarly, we propose here the evolution of a rudimentary CCM (C4-like type pathway) in the non-foliar tissues of the Poaceae, prior to the evolution of the C4 pathway as identified in the leaves of the C4 species of the PACMAD clade.

Similar content being viewed by others
Data availability
The data pertaining to the discussion in this study, developed by the author is available for access in the public domain (E-MTAB-8361 / PRJEB34534).
Code availability
Not applicable.
References
Allakhverdiev SI (2020) Optimising photosynthesis for environmental fitness. Funct Plant Biol 47(11):iii–vii
AuBuchon-Elder T, Coneva V, Goad DM, Jenkins LM, Yu Y, Allen DK, Kellogg EA (2020) Sterile spikelets contribute to yield in sorghum and related grasses. Plant Cell 32:3500–3518
Bast F (2016) Primary succession recapitulates phylogeny. J Phylogenetics Evol Biol 4:e117
Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15(6):330–336
Beer S, Sand-Jensen K, Madsen TV, Nielsen SL (1991) The carboxylase activity of Rubisco and the photosynthetic performance in aquatic plants. Oecologia 87(3):429–434
Bianconi ME, Dunning LT, Moreno-Villena JJ, Osborne CP, Christin P-A (2018) Gene duplication and dosage effects during the early emergence of C4 photosynthesis in the grass genus Alloteropsis. J Exp Bot 69(8):1967–1980
Bort J, Brown R, Araus J (1995) Lack of C4 photosynthetic metabolism in ears of C3 cereals. Plant Cell Environ 18(6):697–702
Brestic M, Zivcak M, Hauptvogel P, Misheva S, Kocheva K, Yang X, Li X, Allakhverdiev SI (2018) Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth Res 136(2):245–255
Cesarino I, Ioio RD, Kirschner GK, Ogden MS, Picard KL, Rast-Somssich MI, Somssich M (2020) Plant science’s next top models. Ann Bot 126:1–23
Christin P-A, Arakaki M, Osborne CP, Edwards EJ (2015) Genetic enablers underlying the clustered evolutionary origins of C4 photosynthesis in angiosperms. Mol Biol Evol 32(4):846–858
Christin P-A, Boxall SF, Gregory R, Edwards EJ, Hartwell J, Osborne CP (2013a) Parallel recruitment of multiple genes into C4 photosynthesis. Genome Biol Evol 5(11):2174–2187
Christin P-A, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ (2013b) Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc Natl Acad Sci 110(4):1381–1386
Christin P-A, Osborne CP, Sage RF, Arakaki M, Edwards EJ (2011) C4 eudicots are not younger than C4 monocots. J Exp Bot 62(9):3171–3181
Christin P-A, Spriggs E, Osborne CP, Strömberg CA, Salamin N, Edwards EJ (2014) Molecular dating, evolutionary rates, and the age of the grasses. Syst Biol 63(2):153–165
Clark JW, Donoghue PC (2018) Whole-genome duplication and plant macroevolution. Trends Plant Sci 23(10):933–945
Crespo H, Frean M, Cresswell C, Tew J (1979) The occurrence of both C 3 and C 4 photosynthetic characteristics in a single Zea mays plant. Planta 147(3):257–263
Dann M, Leister D (2017) Enhancing (crop) plant photosynthesis by introducing novel genetic diversity. Philos Trans R Soc B 372(1730):20160380
Deng X, Liu Y, Xu X, Liu D, Zhu G, Yan X, Wang Z, Yan Y (2018) Comparative proteome analysis of wheat flag leaves and developing grains under water deficit. Front Plant Sci 9:425
Devos N, Szövényi P, Weston DJ, Rothfels CJ, Johnson MG, Shaw AJ (2016) Analyses of transcriptome sequences reveal multiple ancient large-scale duplication events in the ancestor of Sphagnopsida (Bryophyta). New Phytol 211(1):300–318
Dunning LT, Moreno-Villena JJ, Lundgren MR, Dionora J, Salazar P, Adams C, Nyirenda F, Olofsson JK, Mapaura A, Grundy IM (2019) Key changes in gene expression identified for different stages of C4 evolution in Alloteropsis semialata. J Exp Bot 70(12):3255–3268
Edwards EJ, Ogburn RM (2012) Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. Int J Plant Sci 173(6):724–733
Edwards EJ, Osborne CP, Strömberg CA, Smith SA (2010) The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328(5978):587–591
Estep MC, McKain MR, Diaz DV, Zhong J, Hodge JG, Hodkinson TR, Layton DJ, Malcomber ST, Pasquet R, Kellogg EA (2014) Allopolyploidy, diversification, and the Miocene grassland expansion. Proc Natl Acad Sci 111(42):15149–15154
Farmer AM, Maberly SC, Bowes G (1986) Activities of carboxylation enzymes in freshwater macrophytes. J Exp Bot 37(10):1568–1573
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Biol 40(1):503–537
Fawcett JA, Maere S, Van De Peer Y (2009) Plants with double genomes might have had a better chance to survive the Cretaceous-Tertiary extinction event. Proc Natl Acad Sci USA 106(14):5737–5742
Gao B, Chen M, Li X, Zhang J (2019) Ancient duplications and grass-specific transposition influenced the evolution of LEAFY transcription factor genes. Commun Biol 2(1):1–10
Grass Phylogeny Working Group G (2012) New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol 193(2):304–312
Green W (2010) The function of the aerenchyma in arborescent lycopsids: evidence of an unfamiliar metabolic strategy. Proc R Soc B 277(1692):2257–2267
Groenhof AC, Smirnoff N, Bryant JA (1988) Enzymic activities associated with the ability of aerial and submerged forms of Littorella uniflora (L.) Aschers to perform CAM. J Exp Bot 39(3):353–361
Guralnick LJ, Edwards G, Ku MS, Hockema B, Franceschi V (2002) Photosynthetic and anatomical characteristics in the C4–crassulacean acid metabolism-cycling plant Portulaca grandiflora. Funct Plant Biol 29(6):763–773
Han S, Maberly SC, Gontero B, Xing Z, Li W, Jiang H, Huang W (2020) Structural basis for C4 photosynthesis without Kranz anatomy in leaves of the submerged freshwater plant Ottelia alismoides. Ann Bot 125:869–879
Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber AP, Lercher MJ (2013) Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153(7):1579–1588
Henry RJ, Furtado A, Rangan P (2020) Pathways of photosynthesis in non-leaf tissues. Biology 9(12):438
Henry RJ, Rangan P, Furtado A (2016) Functional cereals for production in new and variable climates. Curr Opin Plant Biol 30:11–18
Henry RJ, Rangan P, Furtado A, Busch FA, Farquhar GD (2017) Does C4 photosynthesis occur in wheat seeds? Plant Physiol 174(4):1992–1995
Heyduk K, Moreno-Villena JJ, Gilman IS, Christin P-A, Edwards EJ (2019) The genetics of convergent evolution: insights from plant photosynthesis. Nat Rev Genet 20(8):485–493
Hibberd JM, Covshoff S (2010) The regulation of gene expression required for C4 photosynthesis. Annu Rev Plant Biol 61:181–207
Hibberd JM, Quick WP (2002) Characteristics of C 4 photosynthesis in stems and petioles of C 3 flowering plants. Nature 415(6870):451–454
Hostrup O, Wiegleb G (1991) The influence of different CO2 concentrations in the light and the dark on diurnal malate rhythm and phosphoenolpyruvate carboxylase activities in leaves of Littorella uniflora (L.) Aschers. Aquat Bot 40(1):91–100
Hu L, Zhang Y, Xia H, Fan S, Song J, Lv X, Kong L (2019) Photosynthetic characteristics of non-foliar organs in main C3 cereals. Physiol Plant 166(1):226–239
Huang CH, Qi X, Chen D, Qi J, Ma H (2020) Recurrent genome duplication events likely contributed to both the ancient and recent rise of ferns. J Integr Plant Biol 62(4):433–455
Huang P, Studer AJ, Schnable JC, Kellogg EA, Brutnell TP (2017) Cross species selection scans identify components of C4 photosynthesis in the grasses. J Exp Bot 68(2):127–135
Hussain S, Ulhassan Z, Brestic M, Zivcak M, Zhou W, Allakhverdiev SI, Yang X, Safdar ME, Yang W, Liu W (2021) Photosynthesis research under climate change. Photosynth Res 150(1):5–19
Jansson C, Vogel J, Hazen S, Brutnell T, Mockler T (2018) Climate-smart crops with enhanced photosynthesis. J Exp Bot 69(16):3801–3809
Jones TP (1994) 13C enriched Lower Carboniferous fossil plants from Donegal, Ireland: carbon isotope constraints on taphonomy, diagenesis and palaeoenvironment. Rev Palaeobot Palynol 81(1):53–64
Jung J, Lee SC, Choi H-K (2008) Anatomical patterns of aerenchyma in aquatic and wetland plants. J Plant Biol 51(6):428–439
Kadereit G, Bohley K, Lauterbach M, Tefarikis DT, Kadereit JW (2017) C3–C4 intermediates may be of hybrid origin–a reminder. New Phytol 215(1):70–76
Keeley J (1996) Aquatic CAM photosynthesis. In: Winter K, Smith A (eds) Crassulacean acid metabolism biochemistry, eophysiology and evolution, vol 114. Ecological studies. Springer, USA, pp 281–295
Keeley J (1998a) Diel acid fluctuations in C4 amphibious grasses. Photosynthetica 35(2):273–277
Keeley JE (1981) Isoetes howellii: a submerged aquatic CAM plant? Am J Bot 68(3):420–424
Keeley JE (1998b) CAM photosynthesis in submerged aquatic plants. Bot Rev 64(2):121–175
Keeley JE (1999) Photosynthetic pathway diversity in a seasonal pool community. Funct Ecol 13(1):106–118
Keeley JE, Busch G (1984) Carbon assimilation characteristics of the aquatic CAM plant, Isoetes howellii. Plant Physiol 76(2):525–530
Keeley JE, Osmond CB, Raven JA (1984) Stylites, a vascular land plant without stomata absorbs CO2 via its roots. Nature 310(5979):694–695
Koch K, Kennedy RA (1980) Characteristics of crassulacean acid metabolism in the succulent C4 dicot Portulaca Oleracea l. Plant Physiol 65(2):193–197
Kraybill AA, Martin CE (1996) Crassulacean acid metabolism in three species of the C4 genus Portulaca. Int J Plant Sci 157(1):103–109
Krishnan S, Dayanandan P (2003) Structural and histochemical studies on grain-filling in the caryopsis of rice (Oryza sativa L.). J Biosci 28(4):455–469
Liu M, Pan T, Allakhverdiev SI, Yu M, Shabala S (2020) Crop halophytism: an environmentally sustainable solution for global food security. Trends Plant Sci 25(7):630–634
Ludwig M (2013) Evolution of the C4 photosynthetic pathway: events at the cellular and molecular levels. Photosynth Res 117(1):147–161
Lundgren MR (2020) C2 photosynthesis: a promising route towards crop improvement? New Phytol 228(6):1734–1740
Mallmann J, Heckmann D, Bräutigam A, Lercher MJ, Weber AP, Westhoff P, Gowik U (2014) The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. Elife 3:e02478
Martin W, Scheibe R, Schnarrenberger C (2000) The Calvin cycle and its regulation. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Photosynthesis: physiology and metabolism, vol 9. Advances in photosynthesis. Springer, New York, pp 9–51
Matsuoka M (1995) The gene for pyruvate, orthophosphate dikinase in C4 plants: structure, regulation and evolution. Plant Cell Physiol 36(6):937–943
Matsuoka M, Kyozuka J, Shimamoto K, Kano-Murakami Y (1994) The promoters of two carboxylases in a C4 plant (maize) direct cell-specific, light-regulated expression in a C3 plant (rice). Plant J 6(3):311–319
McKain MR, Tang H, McNeal JR, Ayyampalayam S, Davis JI, Depamphilis CW, Givnish TJ, Pires JC, Stevenson DW, Leebens-Mack JH (2016) A phylogenomic assessment of ancient polyploidy and genome evolution across the Poales. Genome Biol Evol 8(4):1150–1164
McNevin DB, Badger MR, Whitney SM, Von Caemmerer S, Tcherkez GG, Farquhar GD (2007) Differences in carbon isotope discrimination of three variants of d-ribulose-1,5-bisphosphate carboxylase/oxygenase reflect differences in their catalytic mechanisms. J Biol Chem 282(49):36068–36076
Miyagawa Y, Tamoi M, Shigeoka S (2001) Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat Biotechnol 19(10):965–969
Moreno-Villena JJ, Dunning LT, Osborne CP, Christin P-A (2018) Highly expressed genes are preferentially co-opted for C4 photosynthesis. Mol Biol Evol 35(1):94–106
Morrison I (1976) The structure of the chlorophyll-containing cross cells and tube cells of the inner pericarp of wheat during grain development. Bot Gaz 137(1):85–93
Nielsen SL, Gacia E, Sand-Jensen K (1991) Land plants of amphibious Littorella uniflora (L.) Aschers. maintain utilization of CO2 from the sediment. Oecologia 88(2):258–262
Nielsen SL, Sand-Jensen K (1997) Growth rates and morphological adaptations of aquatic and terrestrial forms of amphibious Littorella uniflora (L.) Aschers. Plant Ecol 129(2):135–140
Niklaus M, Kelly S (2018) The molecular evolution of C4 photosynthesis: opportunities for understanding and improving the world’s most productive plants. J Exp Bot 70(1):795–804
Nutbeam AR, Duffus CM (1976) Evidence for C4 photosynthesis in barley pericarp tissue. Biochem Biophys Res Commun 70(4):1198–1203
O’Leary MH (1988) Carbon isotopes in photosynthesis. Bioscience 38(5):328–336
Pagani M, Zachos JC, Freeman KH, Tipple B, Bohaty S (2005) Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science 309(5734):600–603
Pedersen O (2020) Jack of all trades–C4 photosynthesis, CAM and HCO3− use in the same tissue. A commentary on: ‘Structural basis for C4 photosynthesis without Kranz anatomy in leaves of the submerged freshwater plant Ottelia alismoides’. Ann Bot 125(6):iv–vi
Peisker M (1986) Models of carbon metabolism in C3–C4 intermediate plants as applied to the evolution of C4 photosynthesis. Plant Cell Environ 9(8):627–635
Pengelly JJ, Kwasny S, Bala S, Evans JR, Voznesenskaya EV, Koteyeva NK, Edwards GE, Furbank RT, von Caemmerer S (2011) Functional analysis of corn husk photosynthesis. Plant Physiol 156(2):503–513
Poschenrieder C, Fernández JA, Rubio L, Pérez L, Terés J, Barceló J (2018) Transport and use of bicarbonate in plants: current knowledge and challenges ahead. Int J Mol Sci 19(5):1352
Prins H, Elzenga J (1989) Bicarbonate utilization: function and mechanism. Aquat Bot 34(1–3):59–83
Pyankov VI, Voznesenskaya EV, Kuz’min AN, Ku MS, Ganko E, Franceschi VR, Black CC, Edwards GE (2000) Occurrence of C 3 and C 4 photosynthesis in cotyledons and leaves of Salsola species (Chenopodiaceae). Photosynth Res 63(1):69–84
Raghavendra A, Rajendrudu G, Das V (1978) Simultaneous occurrence of C 3 and C 4 photosyntheses in relation to leaf position in Mollugo nudicaulis. Nature 273(5658):143–144
Raghavendra AS, Sage RF (eds) (2011) C4 photosynthesis and related CO2 concentrating mechanisms, vol 32. Advances in photosynthesis and respiration. Springer, Dordrecht
Rangan P, Furtado A, Henry RJ (2016) New evidence for grain specific C 4 photosynthesis in wheat. Sci Rep 6:31721
Raven J (1997) CO2-concentrating mechanisms: a direct role for thylakoid lumen acidification? Plant Cell Environ 20(2):147–154
Raven JA, Beardall J, Giordano M (2014) Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynth Res 121(2):111–124
Raven JA, Cockell CS, De La Rocha CL (2008) The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos Trans R Soc b 363(1504):2641–2650
Ren R, Wang H, Guo C, Zhang N, Zeng L, Chen Y, Ma H, Qi J (2018) Widespread whole genome duplications contribute to genome complexity and species diversity in angiosperms. Mol Plant 11(3):414–428
Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161(2):341–370
Sage RF, Khoshravesh R (2016) Passive CO2 concentration in higher plants. Curr Opin Plant Biol 31:58–65
Sage RF, Monson RK (eds) (1999) C4 plant biology. Academic Press, CA
Shevela D, Björn LO, Govindjee G (2019) Photosynthesis: solar energy for life. World Scientific Publishing, Singapore
Silvera K, Santiago LS, Winter K (2005) Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Funct Plant Biol 32(5):397–407
Simkin AJ, Faralli M, Ramamoorthy S, Lawson T (2020) Photosynthesis in non-foliar tissues: implications for yield. Plant J 101(4):1001–1015
Singal H, Sheoran I, Singh R (1986) In vitro enzyme activities and products of 14 CO2 assimilation in flag leaf and ear parts of wheat (Triticum aestivum L.). Photosynth Res 8(2):113–122
Skelton P, Smith A, Monks N (2002) Cladistics: a practical primer on CD-ROM, vol 1. Cambridge University Press, Cambridge
Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Teisher JK, Clark LG, Barberá P, Gillespie LJ, Zuloaga FO (2017) A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications. J Syst Evol 55(4):259–290
Suissa JS, Green WA (2020) Low atmospheric CO2 levels induce nocturnal carbon accumulation in the lycophyte genus Isoëtes. bioRxiv:820514
Takao K, Shirakura H, Hatakeyama Y, Ueno O (2022) Salt stress induces Kranz anatomy and expression of C4 photosynthetic enzymes in the amphibious sedge Eleocharis vivipara. Photosynth Res. https://doi.org/10.1007/s11120-022-00913-y:1-10
Tambussi EA, Bort J, Guiamet JJ, Nogués S, Araus JL (2007) The photosynthetic role of ears in C3 cereals: metabolism, water use efficiency and contribution to grain yield. Crit Rev Plant Sci 26(1):1–16
Tambussi EA, Maydup ML, Carrión CA, Guiamet JJ, Araus JL (2021) Ear photosynthesis in C3 cereals and its contribution to grain yield: Methodologies, controversies, and perspectives. J Exp Bot 72(11):3956–3970
Tambussi EA, Nogués S, Araus JL (2005) Ear of durum wheat under water stress: water relations and photosynthetic metabolism. Planta 221(3):446–458
Tank DC, Eastman JM, Pennell MW, Soltis PS, Soltis DE, Hinchliff CE, Brown JW, Sessa EB, Harmon LJ (2015) Nested radiations and the pulse of angiosperm diversification: increased diversification rates often follow whole genome duplications. New Phytol 207(2):454–467
Tashima M, Yabiku T, Ueno O (2021) Coleataenia prionitis, a C4-like species in the Poaceae. Photosynth Res 147(2):211–227
Tiley GP, Barker MS, Burleigh JG (2018) Assessing the performance of Ks plots for detecting ancient whole genome duplications. Genome Biol Evol 10(11):2882–2898
Vicentini A, Barber JC, Aliscioni SS, Giussani LM, Kellogg EA (2008) The age of the grasses and clusters of origins of C4 photosynthesis. Glob Change Biol 14(12):2963–2977
Walker N, Smith F, Cathers I (1980) Bicarbonate assimilation by fresh-water charophytes and higher plants: I Membrane transport of bicarbonate ions is not proven. J Membr Biol 57(1):51–58
Wang J-L, Turgeon R, Carr JP, Berry JO (1993) Carbon sink-to-source transition is coordinated with establishment of cell-specific gene expression in a C4 plant. Plant Cell 5(3):289–296
Wang X, Gowik U, Tang H, Bowers JE, Westhoff P, Paterson AH (2009) Comparative genomic analysis of C4 photosynthetic pathway evolution in grasses. Genome Biol 10(6):R68
Wu Y (2021) Is bicarbonate directly used as substrate to participate in photosynthetic oxygen evolution. Acta Geochim 40(4):650–658
Yu Y (2020) Paving the way for C4 evolution: study of C3–C4 intermediate species in grasses. Plant Physiol 182:453–454
Zachos J, Pagani M, Sloan L, Thomas E, Billups K (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292(5517):686–693
Zachos JC, Dickens GR, Zeebe RE (2008) An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451(7176):279–283
Zhang Y, Yin L, Jiang H-S, Li W, Gontero B, Maberly SC (2014) Biochemical and biophysical CO 2 concentrating mechanisms in two species of freshwater macrophyte within the genus Ottelia (Hydrocharitaceae). Photosynth Res 121(2–3):285–297
Acknowledgements
The authors acknowledge the Indian Council of Agricultural Research, New Delhi for the research grant 16113160001-1006976 under the ‘Incentivizing research in Agriculture’ scheme. The authors are grateful to Govindjee Govindjee (of the University of Illinois at Urbana-Champaign, USA) and the anonymous reviewers, for their critical inputs, comments, and suggestions.
Funding
Indian Council of Agricultural Research, New Delhi (16113160001–1006976).
Author information
Authors and Affiliations
Contributions
Investigation and formal analysis, Parimalan Rangan, Rajkumar Subramani, Dhammaprakash Wankhede, Viswanathan Chinnusamy and Kuldeep Singh; Funding acquisition, Surendra Malik and Mirza Jaynul Baig; Supervision, Conceptualization and hypothesis, Parimalan Rangan; Writing – original draft, Parimalan Rangan; Writing – review & editing, Parimalan Rangan, Robert Henry, Dhammaprakash Wankhede, Rajkumar Subramani, Viswanathan Chinnusamy, Surendra Malik, Mirza Jaynul Baig and Kuldeep Singh.
Corresponding author
Ethics declarations
Competing interests
All authors declare that there are no direct or indirect, including financial and non-financial, competing interests, with reference to this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have consented for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rangan, P., Wankhede, D.P., Subramani, R. et al. Evolution of an intermediate C4 photosynthesis in the non-foliar tissues of the Poaceae. Photosynth Res 153, 125–134 (2022). https://doi.org/10.1007/s11120-022-00926-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-022-00926-7