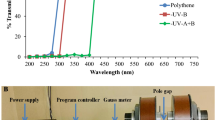
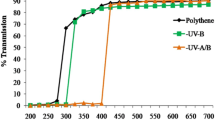
Abstract
The study was performed to analyze the impact of seed pretreatment by static magnetic field (SMF) of 200 mT for 1 h on photosynthetic performance of soybean (Glycine max) seedlings under ambient (aUV-B) and supplemental ultraviolet-B (a+sUV-B) stress. Ambient and supplemental UV-B were found to decrease the plant growth, chlorophyll concentration, PSII efficiency, selected JIP-test parameters such as Fv/Fm, φEo, ΔV(I–P), PIABS, PItotal, and rate of photosynthesis in the leaves of soybean seedlings emerged from untreated (UT) seeds. aUV-B and a+sUV-B were observed to increase the synthesis of UV-B-absorbing substances (UAS), reactive oxygen species (ROS) like superoxide radical (O2·−) and hydrogen peroxide (H2O2), antioxidants like ascorbic acid and α-tocopherol and decrease the nitrate reductase (NR) activity; subsequently, it results in a decreased rate of photosynthesis, biomass accumulation, and yield. However, our results provided evidence that SMF pretreatment increased the tolerance of soybean seedlings to UV-B radiation by increased NO content and NR activity; higher efficiency of PSII, higher values of φEo, ΔV(I–P), PIABS, and PItotal, decreased intercellular CO2 concentration, lower amount of UAS, ROS, and antioxidants that consequently improve the yield of soybean plants under aUV-B as well as a+sUV-B stress. Thus, our results suggested that SMF pretreatment mitigates the adverse effects of UV-B stress by the enhancement in photosynthetic performance along with higher NO content which may be able to protect the plants from the deleterious effects of oxidative stress caused by UV-B irradiation.








Similar content being viewed by others
Abbreviations
- ASA:
-
Ascorbic acid
- Chl:
-
Chlorophyll
- F v/F m :
-
The maximum quantum yield (efficiency) of PS II photochemistry
- FW:
-
Fresh weight
- NO:
-
Nitric oxide
- NR:
-
Nitrate reductase
- φEo:
-
Quantum yield of electron transport
- PIABS :
-
Performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of intersystem electron acceptors
- PItotal :
-
Performance index (potential) for energy conservation from the photons absorbed by PSII to the reduction of photosystem I electron end-acceptors
- ROS:
-
Reactive oxygen species
- aUV-B:
-
Ambient UV-B
- a + sUV-B:
-
Supplemental or enhanced ultraviolet-B
- UAS:
-
UV-B absorbing substances
- ΔV (I–P):
-
Relative amplitude of the I–P phase of Chla fluorescence
References
Ahmad P, Abdel Latef AA, Hashem A, Abd_Allah EF, Gucel S, Tran SP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in Chickpea. Front Plant Sci 7:347. https://doi.org/10.3389/fpls.2016.00347
Ahanger MA, Aziz U, Alsahli AA, Alyemeni MN, Ahmad P (2020) Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules 10(1):42. https://doi.org/10.3390/biom10010042
Albert KR, Mikkelsen TN, Ro-Poulsen H (2005) Effects of ambient versus reduced UV-B radiation on high arctic Salix arctica assessed by measurements and calculations of chlorophyll a fluorescence parameters from fluorescence transients. Physiol Plant 124:208–226. https://doi.org/10.1111/j.1399-3054.2005.00502.x
Allen DJ, Nougúes S, Baker NR (1998) Ozone depletion, and increased UV-B radiation: is there a real threat to photosynthesis? J Exp Bot 49:1775–1778. https://doi.org/10.1093/jxb/49.328.1775
An LZ, Liu YH, Zhang MX (2005) Effect of nitric oxide on growth of maize seedling leaves in the presence or absence of ultraviolet-B radiation. J Plant Physiol 162:317–326. https://doi.org/10.1016/j.jplph.2004.07.004
Akhter MS, Noreen S, Mahmood S, Athar HR, Ashraf M, Abdullah AA, Ahmad P (2021) Influence of salinity stress on PSII in barley (Hordeum vulgare L.) genotypes, probed by chlorophyll-a fluorescence. J King Saud Univ Sci 33:101239. https://doi.org/10.1016/j.jksus.2020.101239
Anand A, Nagarajan S, Verma A, Joshi D, Patha P, Bhardwaj J (2012) pretreatment of seeds with static magnetic field ameliorates soil water stress in seedlings of maize (Zea mays L.). Indian J Biochem Biophys 49:63–70
Arakawa N, Tsutsumi K, Sanceda NG, Kurata T, Inagaki C (1981) A rapid and sensitive method for determination of ascorbic acid using 4,7-Diphenyl-l, 10-bathophenanthroline. Agric Biol Chem 45:1289–1290
Banks JM (2017) Continuous excitation chlorophyll fluorescence parameters: a review for practitioners.Tree Physiol 37:1128–1136. https://doi.org/10.1093/treephys/tpx059
Baghel L, Kataria S, Guruprasad KN (2015) Impact of pre-sowing exposure of seeds to stationary magnetic field on nitrogen and carbon metabolism in maize and soybean. Int J Trop Agric 33:977–983
Baghel L, Kataria S, Guruprasad KN (2018) Effect of SMF pretreatment on growth, photosynthetic performance and yield of soybean under water stress. Photosynthetica 56:718–730
Bais AF, Bernhard G, McKenzie RL, Aucamp PJ, Young PJ, Ilyas M, Jockel P, Deushi M (2019) Ozone–climate interactions and effects on solar ultraviolet radiation. Photochem Photobiol Sci 18:602–640
Balakumar T, Sevakumar V, Sathiameena K, Ilanchezhian CM, Paliwal K (1999) UV-B radiation mediated alterations in the nitrate assimilation pathway of cropplants-1. Kinetic characteristics of nitrate reductase. Photosynthetica 37:459–467
Baroniya SS, Kataria S, Pandey GP, Guruprasad KN (2011) Intraspecific variationin sensitivity to ambient ultraviolet-B radiation in growth and yield characteris-tics of eight soybean cultivars grown under field conditions. Braz J Plant Physiol 23:197–202
Baroniya SS, Kataria S, Pandey GP, Guruprasad KN (2013) Intraspecific variations in antioxidant defense responses and sensitivity of soybean varieties to ambient UV radiation. Acta Physiol Plant 35:1521–1530
Baroniya SS, Kataria S, Pandey GP, Guruprasad KN (2014) Growth, photosynthesis and nitrogen metabolism in soybean varieties after exclusion of the UV-B and UV-A/B components of solar radiation. Crop J 2:388–397
Basahi JM, Ismail IM, Hassan IA (2014) Effects of enhanced UV-B radiation and drought stress on photosynthetic performance of lettuce (Lactuca sativa L. Romaine) plants. Annu Res Rev Biol 4:1739
Bernardini A, Salvatori E, Guerrini V, Fusaro L, Canepari S, Manes F (2015) Effects of high Zn and Pb concentrations on Phragmites australis (Cav.) Trin. Ex. Steudel: photosynthetic performance and metal accumulation capacity under controlled conditions. Int J Phytoremediat 18:16–24. https://doi.org/10.1080/15226514.2015.1058327
Björn LO (2015) Ultraviolet-A, B and C. UV4 Plants Bull 1:17–18
Bornman JF, Barnes PW, Robson TM, Robinson SA, Jansen MAK, Ballaré CL, Flint SD (2019) Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems. Photochem Photobiol Sci 18:681–716. https://doi.org/10.1039/C8PP90061B
Bussotti F, Desotgiu R, Cascio C, Pollastrini M, Gravano E, Gerosa G, Marzuoli C, Lorenzini G, Salvatori E, Manes F, Schaub M, Strasser RJ (2011) Ozone stress in woody plants assessed with chlorophyll a fluorescence. A critical reassessment of existing data. Environ Exp Bot 73:19–30. https://doi.org/10.1016/j.envexpbot.2010.10.022
Bussotti F, Gerosa G, Digrado A, Po llastrini M (2020) Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol Indic 108:105686. https://doi.org/10.1016/j.ecolind.2019.105686
Caldwell MM, Teramura AH, Tevini M, Bornman JF, Bjorn LO, Kulandaivellu G (1995) Effects of increased solar ultraviolet radiation on terrestrial plants. Ambio 24:166–173
Caldwell MM, Bornman JF, Ballaré CL, Flint SD, Kulandaivelu G (2007) Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem Photobiol Sci 6:252–266
Canovas FM, Avila C, Canton FR, Canas RA, de la Torre F (2007) Ammonium assimilation and amino acid metabolism in conifers. J Exp Bot 58:2307–2318
Cascio C, Schaub M, Novak K, Desotgiu R, Bussotti F, Strasser RJ (2010) Foliar responses to ozone of Fagus sylvatica L. seedlings grown in shaded and in full sunlight conditions. Environ Exp Bot 68:188–197. https://doi.org/10.1016/j.envexpbot.2009.10.003
Chaitanya KS, Naithani SC (1994) Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Gaertn.f. New Phytol 126:623–627
Chen L, Zhang S (2007) Effects of enhanced ultraviolet-B radiation on water use efficiency, stomatal conductance, leaf nitrogen content, and morphological characteristics of Spiraea pubescens in a warm-temperate deciduous broad-leaved forest. Front For China 2:401. https://doi.org/10.1007/s11461-007-0064-6
Chen JJ, Zu YQ, Chen HY, Li Y (2004) Influence of enhanced UV-B radiation on growth and biomass allocation of twenty soybean cultivars. J Agro-Environ Sci 23:29–33
Day TA, Neale PJ (2002) Effects of UV-B radiation on terrestrial and aquatic primary producers. Annu Rev Ecol Syst 33:371–396
Dwivedi R, Singh VP, Kumar J, Prasad SM (2015) Differential physiological and biochemical responses of two Vigna species under enhanced UV-B radiation. J Radiat Res Appl Sci 8:173–181
Fatima A, Kataria S, Prajapati R, Jain M, Agrawal AK, Singh B, Kashyap Y, Tripathi DK, Singh VP, Gadre R (2020) Magnetopriming effects on arsenic stress-induced morphological and physiological variations in soybean involving synchrotron imaging. Physiol Plant. https://doi.org/10.1111/ppl.13211
Ghisi R, Trentin AR, Masi A, Ferretti M (2002) Carbon and nitrogen metabolism in barley plants exposed to UV-B radiation. Physiol Plant 116:200–205
Greenberg BM, Wilson MI, Huang XD, Duxbury CL, Gerhaddt KE, Gensemer RW (1997) The effects of ultraviolet- B radiation on higher plants. In: Wang W, Goursuch J, Hughes JS (eds) Plants for environmental studies. CRC Press, Boca Raton, pp 1–35
Hasanuzzaman M, Hossain MA, da Silva J, Fujita M (2012) Plant response and tolerance to abiotic oxidative stress, antioxidant defenseis a key factor. In: Venkateswarlu B, Shanker AK, Shanker C, Maheswari M (eds) Crop stress and its management, perspectives and strategies. Springer, Rijeka, pp 261–315
He J, Huang LK, Chow WS, Whitecross MI, Anderson JM (1994) Responses of rice and pea plants to hardening with low doses of ultraviolet-B radiation. Aust J Plant Physiol 21:563–574
Hiscox J, Israelstam G (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Hopkins L, Bond MA, Tobin AK (2002) Ultraviolet-B radiation reduces the rates of cell division and elongation in the primary leaf of wheat (Triticum aestivum L. cv Maris Huntsman). Plant Cell Environ 25:617–624
Hunt R (1982) Plant growth analysis. University Press, Baltimore, USA
Inostroza-Blancheteau C, Reyes-Díaz M, Arellano A, Latsague M, Acevedo P, Loyola R, Arce-Johnson P, Alberdi M (2014) Effects of UV-B radiation on anatomical characteristics, phenolic compounds and gene expression of the phenylpropanoid pathway in highbush blueberry leaves. Plant Physiol Biochem 85:85–95
Jain K, Kataria S, Guruprasad KN (2004) Oxyradicals under UV-B stress and their quenching by antioxidants. Indian J Exp Biol 42:884–892
Jansen AK, Gaba V, Greenberg BM (1998) Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci 3:131–135
Jaworski EG (1971) Nitrate reductase assay in intact plant tissue. Biochem Biophys Res Commun 43:1274–1279
Jenkins GI (2009) Signal transduction in response to UV-B radiation. Annu Rev Plant Biol 60:407–431. https://doi.org/10.1146/annurev.arplant.59.032607.092953
Jordan BR, He J, Chow WS, Anderson JM (1992) Changes in mRNA levels and polypeptide subunits of ribulose-1,5-bisphosphate carboxylase in response to supplemental UV-B radiation. Plant Cell Environ 15:91–98
Jordan BR, Strid Å, Wargent JJ (2016) What role does UV-B play in determining photosynthesis? In: Pessarakli M (ed) Handbook of photosynthesis. CRC Press, Boca Raton, pp 275–286
Kalaji HM, Schansker G, Ladle RJ et al (2014) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res 122:121–158. https://doi.org/10.1007/s11120-014-0024-6
Kalaji MH, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska AI, Cetner DM, Lukasik I, Goltsev V, Ladle JR (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102
Kalaji HM, Rastogi A, Živčák M et al (2018) Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica 56:953–961. https://doi.org/10.1007/s11099-018-0766-z
Kakani VG, Reddy KR, Zhao D, Mohammed AR (2003a) Effects of ultraviolet-B radiation on cotton (Gossypium hirsutum L.) morphology and anatomy. Ann Bot 91:817–826
Kakani VG, Reddy KR, Zhao D, Sailaja K (2003b) Field crop responses to ultraviolet-B radiation: a review. Agric For Meteorol 120:191–218. https://doi.org/10.1016/j.agrformet.2003.08.015
Kakani VG, Reddy KR, Zhao D, Goa W (2004) Senescence and hypospectral reflectance of cotton leaves exposed to ultraviolet radiation and carbon dioxide. Physiol Plant 221:250–257
Kataria S, Guruprasad KN (2018) Interaction of cytokinins with UV-B (280–315 nm) on the expansion growth of cucumber cotyledons. Hortic Int J 2(2):45–53
Kataria S, Jain K, Guruprasad KN (2007) UV-B induced changes in antioxidant enzymes and their isoforms in cucumber (Cucumis sativus L.) .cotyledons. Ind J Biochem Biophys 44:31–37
Kataria S, Guruprasad KN, Ahuja S, Singh B (2013) Enhancement of growth, photosynthetic performance and yield by the exclusion of ambient UV components in C3 and C4 plants. Photochem Photobiol B Biol 127:140–152
Kataria S, Jajoo A, Guruprasad KN (2014a) Impact of increasing ultraviolet-B radiation on photosynthetic processes. J Photochem Photobiol B 137:55–66
Kataria S, Baroniya S, Baghel L, Kanungo M (2014b) Effect of exclusion of solar UV radiation on plants. Plant Sci Today 1:224–232. https://doi.org/10.14719/pst.2014.1.4.61
Kataria S, Baghel L, Guruprasad KN (2017) Alleviation of adverse effects of ambient UV stress on growth and some potential physiological attributes in soybean (Glycine max) by seed pretreatment with static magnetic field. J Plant Growth Regul 36:550–565
Kataria S, Baghel L, Jain M, Guruprasad KN (2019) Magnetopriming regulates antioxidant defense system in soybean against salt stress. Biocatal Agric Biotechnol 18:101090
Kataria S, Rastogi A, Bele A, Jain M (2020) Role of nitric oxide and reactive oxygen species in static magnetic field pretreatment induced tolerance to ambient UV-B stress in soybean. Physiol Mol Biol Plants 26:939–945. https://doi.org/10.1007/s12298-020-00802-5
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020a) Nitrate reductase rather than nitric oxide synthase activity is involved in 24-epibrassinolide-induced nitric oxide synthesis to improve tolerance to iron deficiency in strawberry (Fragaria × annassa) by up-regulating the ascorbate-glutathione cycle. Plant Physiol Biochem 151:486–499
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020b) The role of nitrate reductase in brassinosteroid-induced endogenous nitric oxide generation to improve cadmium stress tolerance of pepper plants by up-regulating the ascorbate-glutathione cycle. Ecotoxicol Environ Saf 196:110483. https://doi.org/10.1016/j.ecoenv.2020.110483
Krupa SV, Kickert RN (1989) The greenhouse effect: impacts of ultraviolet (UV)-B radiation, carbon dioxide (CO2) and ozone (O3) on vegetation. Environ Pollut 61:263–392
Kubiś J, Rybus-Zając M (2008) Drought and excess UV-B irradiation differentially alter the antioxidant system in cucumber leaves. Acta Biol Crac Ser Bot 50:35–41
Liu B, Liu X-B, Yan-Sheng L, Herbert SJ (2013) Effects of enhanced UV-B radiation on seed growth characteristicsand yield components in soybean. Field Crop Res 154:158–163
Malanga G, Calmanovici G, Puntarulo S (1997) Oxidative damage to chloroplast from Chlorella vulgaris exposed to ultraviolet-B radiation. Physiol Plant 101:455–462
Martinez-Luscher J, Morales F, Delrot S, Sanchez-Diaz M, Gomes E, Aguirreolea J, Pascual I (2013) Short and long-term physiological responses of grapevine leaves to UV-B radiation. Plant Sci 213:114–122
Mazza CA, Batista D, Zima AM, Szwarcberg-Bracchitta M, Giordano CV, Acevedo A, Scopel AL, Ballare CL (1999) The effects of solar UV-B radiation on the growth and yield of barley are accompanied by increased DNA damage and antioxidant responses. Plant Cell Environ 22:61–70
Mohammed AR, Tarpley L (2010) Differential response of Southern US rice (Oryza sativa L.) cultivars to Ultraviolet-B radiation. J Agron Crop Sci 196:286–295
Mosadegh H, Trivellini A, Ferrante A, Lucchesini M, Vernieri P, Mensuali A (2018) Applications of UV-B lighting to enhance phenolic accumulation of sweet basil. Sci Hortic 229:107–116
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21:31–57
Nikiforou C, Manetas Y (2011) Inherent nitrogen deficiency in Pistacia lentiscus preferentially affects photosystem I: a seasonal field study. Funct Plant Biol 38:848–855
Nogues S, Baker NR (1995) Evaluation of the role of damage to photosystem II in the inhibition of CO2 assimilation in pea leaves on exposure to UV-B radiation. Plant Cell Environ 18:781–787
Oukarroum A, Bussotti F, Goltsev V, Kalaji HM (2015) Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ Exp Bot 109:80–88. https://doi.org/10.1016/j.envexpbot.2014.08.005
Prajapati R, Kataria S, Jain M (2020) Seed priming for alleviation of heavy metal toxicity in plants: an overview. Plant Sci Today 7:16. https://doi.org/10.14719/pst.2020.7.2.751
Pearson CK, Davies RR, Barnes MM (1970) Separation of alphatocotrienol from alpha-tocopherol by polyehtylenecelite column chromatography. Chem Ind 8:275–276
Pollastrini M, Desotgiu R, Camin F, Ziller L, Gerosa G, Marzuoli R, Bussotti F (2014) Severe drought events increase the sensitivity to ozone on poplar clone. Environ Exp Bot 100:94–104. https://doi.org/10.1016/j.envexpbot.2013.12.016
Qiang WY, Yang H, Chen T, An LZ, Wang XL (2004) Effect of the combination of cadmium and UV-B radiation on soybean growth. Chin J Appl Ecol 15:697–700
Quaggiotti S, Trentin AR, Dalla Vecchia F, Ghisi R (2004) Response of maize nitrate reductase to UV-B radiation. Plant Sci 167:107–116
Rao MV, Ormrod DP (1995) Impact of UV-B and O3 on the free radical scavenging in Arabidopsis thaliana genotypes differing in flavonoid biosynthesis. Photochem Photobiol 62:719–726
Rastogi A, Pospisil P (2013) Ultra-weak photon emission as a non-invasive tool for the measurement of oxidative stress induced by UVA radiation in Arabidopsis thaliana. J Photochem Photobiol B Biol 123:59–64
Rastogi A, Yadav D, Szymańska R et al (2014) Singlet oxygen scavenging activity of tocopherol and plastochromanol in Arabidopsis thaliana: relevance to photooxidative stress. Plant Cell Environ 37(2):392–401
Rastogi A, Stróżecki M, Kalaji HM, Łuców D, Lamentowicz M, Juszczak R (2019a) Impact of warming and reduced precipitation on photosynthetic and remote sensing properties of peatland vegetation. Environ Exp Bot 160:71–80
Rastogi A, Zivcak M, Tripathi DK, Yadav S, Kalaji HM, Brestic M (2019b) Phytotoxic effect of silver nanoparticles in Triticum aestivum: improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 57(1):209–216
Rastogi A, Kovar M, He X, Zivcak M, Kataria S, Kalaji HM, Skalicky M, Ibrahimova UF, Hussain S, Mbarki S, Brestic M (2020) Special issue in honour of Prof. Reto J Strasser—JIP-test as a tool to identify salinity tolerance in sweet sorghum genotypes. Photosynthetica 58:518–528
Reddy KR, Kakanl VG, Zhao D, Kotl S, Gao W (2004) Interactive effects of ultraviolet-B radiation and temperature on cotton physiology, growth, development and hyperspectral reflectance. Photochem Photobiol 79:416–427
Redillas MC, Jeong JS, Strasser RJ, Kim YS, Kim JK (2011) JIP analysis on rice (Oryza sativa cv. Nipponbare) grown under limited nitrogen conditions. J Korean Soc Appl Biol Chem 54:827–832
Ripley BS, Redfern SP, Dames JF (2004) Quantification of the photosynthetic performance of phosphorus-deficient Sorghum by means of chlorophyll-a fluorescence kinetics. S Afr J Sci 100:615–618
Robson M, Klema K, Urban O, Jansen M (2015) Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ 38:856–866. https://doi.org/10.1111/pce.12374
Rockel P (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110. https://doi.org/10.1093/jexbot/53.366.103
Ros J, Tevini M (1995) Interaction of UV-radiation and IAA during growth of seedlings and hypocotyls segments of sunflower. J Plant Physiol 146:295–302
Rowland F (2006) Stratospheric ozone depletion. Philos Trans Biol Sci 361(1469):769–790
Rybus-Zając M (2005) Oxidative stress generation in Taxus baccata leaves affected by Pestalotiopsis funerea Desm. under different light conditions. Dendrobiology 54:51–56
SakakiT KN, Sugahara K (1983) Breakdown of photosynthetic pigments and lipids in spinach leaves with ozone fumigation: role of activated oxygen. Physiol Plant 9:28–34
Santos VA, Nelson BW, Rodrigues JV, Garcia MN, Ceron VB, Ferreira MJ (2019) Fluorescence parameters among leaf photosynthesis-related traits are the best proxies for CO2 assimilation in Central Amazon trees. Braz J Bot 42:239–247. https://doi.org/10.1007/s40415-019-00533-2
Schansker G, Toth SZ, Strasser RJ (2006) Dark-recovery of the Chl a fluorescence transient (OJIP) after light adaptation: the qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim Biophys Acta 1757:787–797
Schansker G, Tóth SZ, Strasser RJ (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochimica et Biophysica Acta 1706:250–261
Searles PS, Flint SD, Caldwell MM (2001) A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia 127:1–10
Shah T, Latif S, Saeed F, Ali I, Ullah S, Abdullah A, Jan S, Ahmad P (2020) Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J King Saud Univ Sci. https://doi.org/10.1016/j.jksus.2020.10.004
Shine MB, Guruprasad KN (2012) Oxyradicals and PS II activity in maize leaves in the absence of UV components of the solar spectrum. J Biosci 37:703–712
Shine MB, Guruprasad KN, Anand A (2011) Enhancement of germination, growth, and photosynthesis in soybean by pretreatment of seeds with a magnetic field. Bioelectromagnetics 32:474–484
Shine MB, Guruprasad KN, Anand A (2012) Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics 33:428–437
Singh S, Kumari R, Agrawal M, Agrawal SB (2012) Differential response of radish plants to supplemental ultraviolet-B radiation under varying NPK levels: chlorophyll fluorescence, gas exchange and antioxidants. Physiol Plant 145:474–484. https://doi.org/10.1111/j.1399-3054.2012.01589.x
Singh S, Agrawal M, Agrawal SB (2014) Impact of ultraviolet-B radiation on photosynthetic capacity, antioxidative potential and metabolites in Solanum tuberosum L. under varying levels of soil NPK. Acta Physiol Plant 36:1441–1453. https://doi.org/10.1007/s11738-014-1522-z
Snyrychova I, Kos PB, Hideg E (2007) Hydroxyl radicals are not the protagonists of UV-B induced damage in isolated thylakoid membranes. Funct Plant Biol 34:1112–1121
Stirbet A, Lazár D, Kromdijk J, Govindjee G (2018) Chlorophyll a fluorescence induction: can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 56:86–104. https://doi.org/10.1007/s11099-018-0770-3
Strasser RJ, Tsimilli-Micheal M, Srivastava A (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohnanty P (eds) Probing photosynthesis: mechanisms regulation and adaptation. Taylor and Francis, London, UK, pp 445–483
Suchar VA, Robberecht R (2015) Integration and scaling of UV-B radiation effects on plants: from DNA to leaf. Ecol Evol 5:2544–2555
Sytar O, Kumari P, Yadav S et al (2019) Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul 38:739–752. https://doi.org/10.1007/s00344-018-9886-8
Sztatelman O, Grzy J, Gabrys H, Banas AK (2015) The effect of UV-B on Arabidopsis leaves depends on light conditions after treatment. BMC Plant Biol 15:1–16
Tiitto R, Nenadis N, Neugart S, Robson M, Agati G, Vepsäläinen J, Zipoli G, Nybakken L, Winkler B, Jansen AKM (2015) Assessing the response of plant flavonoids to UV radiation: an overview of appropriate techniques. Phytochem Rev 14:273–297. https://doi.org/10.1007/s11101-014-9362-4
Thomas S, Anand A, Chinnusamy V, Dahuja A, Basu S (2013) Magnetopriming circumvents the effect of salinity stress on germination in chickpea seeds. Acta Physiol Plant 35:3401–3411. https://doi.org/10.1007/s11738-013-1375-x
Vanhaelewyn L, Prinsen E, Van der Straeten D, Vandenbussche F (2016) Hormone, controlled UV-B responses in plants. J Exp Bot 67(15):4469–4482
Wellburn A, Lichtenthaler H (1984) Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In: Advances in photosynthesis research. Springer, pp 9–12
Yu GH, Li W, Yuan ZY, Cui HY, Lv CG, Gao ZP, Han B, Gong YZ, Chen GX (2013) The effects of enhanced UV-B radiation on photosynthetic and biochemical activities in super high-yield hybrid rice Liangyoupeijiu at the reproductive stage. Photosynthetica 51:33–44
Zhang WJ, Björn LO (2009) The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia 80:207–218. https://doi.org/10.1016/j.fitote.2009.02.006
Zhang M, Dong JF, Jin HH, Sun LN, Xu MJ (2011) Ultraviolet-B-induced flavonoid accumulation in Betula pendula leaves is dependent upon nitrate reductase-mediated nitric oxide signaling. Tree Physiol 31:798–807. https://doi.org/10.1093/treephys/tpr070
Zhang M, An L, Feng P, Chen T, Chen K, Liu Y, Tang H, Chang J, Wang X (2003) The cascade mechanisms of nitric oxide as a second messenger of ultraviolet-B ininhibiting mesocotyl elongations. Photochem Photobiol 77:219–225
Zhao D, Reddy KR, Kakani VG, Reed J, Sullivan J (2003) Growth and physiological responses of cotton (Gossypium hirsutum L.) to elevated carbon dioxide and ultraviolet-B radiation under controlled environment conditions. Plant Cell Environ 26:771–782
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Živčák M, Olšovská K, Slamka P et al (2014a) Measurements of chlorophyll fluorescence in different leaf positions may detectnitrogen deficiency in wheat. Zemdirb Agric 101:437–443
Živčák M, Olšovská K, Slamka P et al (2014b) Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ 60:210–215
Živčák M, Brestic M, Kunderlikova K, Sytar O, Allakhverdiev SI (2015) Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth Res. https://doi.org/10.1007/s11120-015-0121-1
Zivcak M, Bruckova K, Sytar O, Brestic M, Olsovska K, Allakhverdiev SI (2017) Lettuce flavonoids screening and phenotyping by chlorophyll fluorescence excitation ratio. Planta 245(6):1215–1229. https://doi.org/10.1007/s00425-017-2676-x
Zuk-Golaszewska K, Upadhyaya MK, Golaszewski J (2003) The effect of UV-B radiation on plant growth and development. Plant Soil Environ 49:135–140
Acknowledgements
Financial assistance by Women Scientists Scheme-A (SR/WOS-A/LS-17/2017) of Department and Science Technology, New Delhi to Dr Sunita Kataria is thankfully acknowledged. Special thanks to Dr. Anjana Jajoo, Head School of Biotechnology, D.A.V.V., Indore, India, for proving the facility of handy PEA fluorimeter (Plant Efficiency Analyzer, Hansatech Instruments, Norfolk, England, UK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kataria, S., Jain, M., Rastogi, A. et al. Static magnetic field treatment enhanced photosynthetic performance in soybean under supplemental ultraviolet-B radiation. Photosynth Res 150, 263–278 (2021). https://doi.org/10.1007/s11120-021-00850-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-021-00850-2