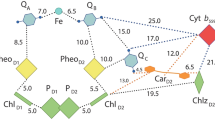
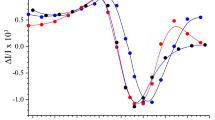
Abstract
Cytb559 in Photosystem II is a heterodimeric b-type cytochrome. The subunits, PsbE and PsbF, consist each in a membrane α-helix. Mutants were previously designed and studied in Thermosynechococcus elongatus (Sugiura et al., Biochim Biophys Acta 1847:276–285, 2015) either in which an axial histidine ligand of the haem-iron was substituted for a methionine, the PsbE/H23M mutant in which the haem was lacking, or in which the haem environment was modified, the PsbE/Y19F and PsbE/T26P mutants. All these mutants remained active showing that the haem has no structural role provided that PsbE and PsbF subunits are present. Here, we have carried on the characterization of these mutants. The following results were obtained: (i) the Y19F mutation hardly affect the Em of Cytb559, whereas the T26P mutation converts the haem into a form with a Em much below 0 mV (so low that it is likely not reducible by QB−) even in an active enzyme; (ii) in the PsbE/H23M mutant, and to a less extent in PsbE/T26P mutant, the electron transfer efficiency from QA− to QB is decreased; (iii) the lower Em of the QA/QA− couple in the PsbE/H23M mutant correlates with a higher production of singlet oxygen; (iv) the superoxide and/or hydroperoxide formation was not increased in the PsbE/H23M mutant lacking the haem, whereas it was significantly larger in the PsbE/T26P. These data are discussed in view of the literature to discriminate between structural and redox roles for the haem of Cytb559 in the production of reactive oxygen species.





Similar content being viewed by others
Abbreviations
- Chl:
-
Chlorophyll
- ChlD1/ChlD2 :
-
Accessory Chl on the D1 or D2 side, respectively
- Cyt:
-
Cytochrome
- DCBQ:
-
2,6-Dichloro-p-benzoquinone
- DCMU:
-
3-(3,4-Dichlorophenyl)-1,1-dimethylurea
- E m :
-
Midpoint redox potential versus SHE, the standard hydrogen electrode
- HP, IP, LP forms:
-
High-potential, intermediate-potential and low-potential forms of Cytb559
- MES:
-
2–(N–morpholino) ethanesulfonic acid
- P680 :
-
Primary electron donor
- PD1 and PD2 :
-
Chl monomer of P680 on the D1 or D2 side, respectively
- Pheo:
-
Pheophytin
- PSII:
-
Photosystem II
- P680 :
-
Chlorophyll dimer acting as the electron donor
- 4-POBN:
-
α-(4-Pyridyl N-oxide)-N-tert-butylnitrone
- QA :
-
Primary quinone acceptor
- QB :
-
Secondary quinone acceptor
- T. elongatus :
-
Thermosynechococcus elongatus
- ROS:
-
Reactive oxygen species
- TL:
-
Thermoluminescence
- WT*3:
-
T. elongatus mutant strain containing only the psbA3 gene
References
Askerka M, Brudvig GW, Batista VS (2017) The O2-evolving complex of photosystem II: recent insights from quantum mechanics/molecular mechanics (QM/MM), extended X-ray absorption fine structure (EXAFS), and femtosecond X-ray crystallography data. Acc Chem Res 50:41–48
Bondarava N, Gross CM, Mubarakshina M, Golecki JR, Johnson GN, Krieger-Liszkay A (2010) Putative function of cytochrome b 559 as a plastoquinol oxidase. Physiol Plant 138:463–473
Buchta J, Grabolle M, Dau H (2007) Photosynthetic dioxygen formation studied by time-resolved delayed fluorescence measurements - Method, rationale, and results on the activation energy of dioxygen formation. Biochim Biophys Acta 1767:565–574
Chiu Y-F, Lin W-C, Wu C-M, Chen Y-H, Hung C-H, Ke S-C, Chu H-A (2009) Identification and characterization of a cytochrome b 559 Synechocystis 6803 mutant spontaneously generated from DCMU-inhibited photoheterotrophical growth conditions. Biochim Biophys Acta 1787:1179–1188
Chiu Y-F, Chen Y-H, Roncel M, Dilbeck PL, Huang J-Y, Ke SC, Ortega JM, Burnap RL, Chu H-A (2013) Spectroscopic and functional characterization of cyanobacterium Synechocystis PCC 6803 mutants on the cytoplasmic-side of cytochrome b 559 in photosystem II. Biochim Biophys Acta 1827:507–519
Cox N, Messinger J (2013) Reflections on substrate water and dioxygen formation. Biochim Biophys Acta 1827:1020–1030
Cser K, Vass I (2007) Radiative and non-radiative charge recombination pathways in Photosystem II studied by thermoluminescence and chlorophyll fluorescence in the cyanobacterium Synechocystis 6803. Biochim Biophys Acta 1767:233–243
Dau H, Zaharieva I, Haumann M (2012) Recent developments in research on water oxidation by photosystem II. Curr Op Chem Biol 16:3–10
Diner BA, Rappaport F (2002) Structure, dynamics, and energetic of the primary photochemistry of photosystem II of oxygenic photosynthesis. Annu Rev Plant Biol 53:551–580
Ducruet JM (2003) Chlorophyll thermoluminescence of leaf discs: simple instruments and progress in signal interpretation open the way to new ecophysiological indicators. J Exp Bot 54:2419–2430
Ducruet JM, Vass I (2009) Thermoluminescence: experimental. Photosynth Res 101:195–204
Guerrero F, Zurita JL, Roncel M, Kirilovsky D, Ortega JM (2014) The role of the high potential form of the cytochrome b 559: study of Thermosynechococcus elongatus mutants. Biochim Biophys Acta 1837:908–919
Hamilton ML, Franco E, Deák Z, Schlodder E, Vass I, Nixon PJ (2014) Investigating the photoprotective role of cytochrome b-559 in photosystem II in a mutant with altered ligation of the haem. Plant Cell Physiol 55:1276–1285
Hung C-H, Huang J-Y, Chiu Y-F, Chu H-A (2007) Site-directed mutagenesis on the heme axial-ligands of cytochrome b 559 in photosystem II by using cyanobacteria Synechocystis PCC 6803. Biochim Biophys Acta 1767:686–693
Hung C-H, Hwang H-J, Chen Y-H, Chiu Y-F, Ke S-C, Burnap RL, Chu H-A (2010) Spectroscopic and functional characterizations of cyanobacterium Synechocystis PCC 6803 mutants on and near the heme axial ligand of cytochrome b 559 in photosystem II. J Biol Chem 285:5653–5663
Ikeuchi M, Inoue Y (1988) A new 4.8-kDa polypeptide intrinsic to the PS II reaction center, as revealed by modified SDS-PAGE with improved resolution of low-molecular-weight proteins. Plant Cell Physiol 29:1233–1239
Joliot P, Kok B (1975) Oxygen evolution in photosynthesis. In: Govindjee (ed) Bioenergetics of photosynthesis. Academic Press, New York, pp 387–412
Kaminskaya OP, Shuvalov VA (2013a) Biphasic reduction of cytochrome b 559 by plastoquinol in photosystem II membrane fragments. Evidence for two types of cytochrome b 559/plastoquinone redox equilibria. Biochim Biophys Acta 1827:471–483
Kaminskaya OP, Shuvalov VA (2013b) Towards an understanding of the nature of the redox forms of cytochrome b559 in photosystem II. Dokl Biochem Biophys 450:151–154
Kern J, Loll B, Lüneberg C, DiFiore D, Biesiadka J, Irrgang K-D, Zouni A (2005) Purification, characterisation and crystallisation of photosystem II from Thermosynechococcus elongatus cultivated in a new type of photobioreactor. Biochim Biophys Acta 1706:147–157
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution–I. A linear four step mechanism. Photochem Photobiol 11:457–475
Ma J-J, Li L-B, Jing Y-X, Kuang T-Y (2007a) Mutation of residue arginine18 of cytochrome b 559 α-subunit and its effects on photosystem II activities in Chlamydomonas reinhardtii. J Integr Plant Biol 49:1054–1061
Ma J-J, Li L-B, Jing Y-X, Kuang T-Y (2007b) Mutagenesis of Ser(24) of cytochrome b 559 alpha subunit affects PSII activities in Chlamydomonas reinhardtii. Chin Sci Bull 52:896–902
Mayhew SG (1978) The redox potential of dithionite and SO2 − from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. Eur J Biochem 85:535–547
Michelet L, Roach T, Fischer BB, Bedhomme M, Lemaire SD, Krieger-Liszkay A (2013) Down-regulation of catalase activity allows transient accumulation of a hydrogen peroxide signal in Chlamydomonas reinhardtii. Plant Cell Environ 36:1204–1213
Morais F, Kühn K, Stewart DH, Barber J, Brudvig GW, Nixon PJ (2001) Photosynthetic water oxidation in cytochrome b 559 mutants containing a disrupted heme-binding pocket. J Biol Chem 276:31986–31993
Müh F, Glöckner C, Hellmich J, Zouni A (2012) Light-induced quinone reduction in photosystem II. Biochim Biophys Acta 1817:44–65
Müh F, Plöckinger M, Renger T (2017) Electrostatic asymmetry in the reaction center of photosystem II. J Phys Chem Lett 8:850–858
Pakrasi HB, De Ciechi P, Whitmarsh J (1991) Site directed mutagenesis of the heme axial ligands of cytochrome b 559 affects the stability of the photosystem II complex. EMBO J 10:1619–1627
Pospíšil P (2011) Enzymatic function of cytochrome b 559 in photosystem II. J Photochem Photobiol B 104:341–347
Pospíšil P (2012) Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim Biophys Acta 1817:218–231
Pospsil P, Snyrychova I, Kruk J, Strzalka K, Naus J (2006) Evidence that cytochrome b 559 is involved in superoxide production in photosystem II: effect of synthetic short-chain plastoquinones in a cytochrome b 559 tobacco mutant. Biochem J 397:321–327
Rappaport F, Lavergne J (2009) Thermoluminescence: theory. Photosynth Res 101:205–216
Rehman AU, Cser K, Sass L, Vass I (2013) Characterization of singlet oxygen production and its involvement in photodamage of photosystem II in the cyanobacterium Synechocystis PCC 6803 by histidine-mediated chemical trapping. Biochem Biophs Acta 1827:689–698
Renger G (2011) Light–induced oxidative water splitting in photosynthesis: energetics, kinetics, and mechanism. J Photochem Photobiol B 104:35–43
Roncel M, Ortega JM, Losada M (2001) Factors determining the special redox properties of photosynthetic cytochrome b 559. Eur J Biochem 268:4961–4968
Roncel M, Boussac A, Zurita JL, Bottin H, Sugiura M, Kirilovsky D, Ortega JM (2003) Redox properties of the photosystem II cytochromes b 559 and c 550 in the cyanobacterium Thermosynechococcus elongatus. J Biol Inorg Chem 8:206–216
Roncel M, Kirilovsky D, Guerrero F, Serrano A, Ortega JM (2012) Photosynthetic cytochrome c 550. Biochim Biophys Acta 1817:1152–1163
Rutherford AW, Krieger-Liszkay A (2001) Herbicide-induced oxidative stress in photosystem II. TIBS 26:648–653
Sedoud A, Kastner L, Cox N, El-Alaoui S, Kirilovsky D, Rutherford AW (2011) Effects of formate binding on the quinone–iron electron acceptor complex of photosystem II. Biochim Biophys Acta 1807:216–226
Shen J-R (2015) The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48
Shinopoulos KE, Brudvig GW (2012) Cytochrome b 559 and cyclic electron transfer within photosystem II. Biochim Biophys Acta 1817:66–75
Stewart DH, Brudvig GW (1998) Cytochrome b 559 of photosystem II. Biochim Biophys Acta 1367:63–68
Suga M, Akita F, Hirata K, Ueno G, Murakami H, Nakajima Y, Shimizu T, Yamashita K, Yamamoto M, Ago H, Shen J-R (2015) Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 517:99–103
Sugiura M, Inoue Y (1999) Highly purified thermo-stable oxygen-evolving photosystem II core complex from the thermophilic cyanobacterium Synechococcus elongatus having His-tagged CP43. Plant Cell Physiol 40:1219–1231
Sugiura M, Boussac A, Noguchi T, Rappaport F (2008) Influence of histidine-198 of the D1 subunit on the properties of the primary electron donor, P680, of photosystem II in Thermosynechococcus elongatus. Biochim Biophys Acta 1777:331–342
Sugiura M, Kato Y, Takahashi R, Suzuki H, Watanabe T, Noguchi T, Rappaport F, Boussac A (2010) Energetics in Photosystem II from Thermosynechococcus elongatus with a D1 protein encoded by either the psbA 1 or psbA 3 gene. Biochim Biophys Acta 1797:1491–1499
Sugiura M, Nakamura M, Koyama K, Boussac A (2015) Assembly of oxygen-evolving photosystem II efficiently occurs with the apo-Cytb 559 but the holo-Cytb 559 accelerates the recovery of functional enzyme upon photoinhibition. Biochim Biophys Acta 1847:276–285
Telfer A (2014) Singlet oxygen production by PSII under light stress: mechanism, detection and the protective role of β-Carotene. Plant cell Physiol 55:1216–1223
Telfer A, Steven MB, Phillips D, Barber J (1994) Isolated photosynthetic reaction center of photosystem II as a sensitizer for the formation of singlet oxygen. J Biol Chem 269(18):13244–13253
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60
Yano J, Yachandra V (2014) Mn4Ca cluster in photosynthesis: where and how water is oxidized to dioxygen. Chem Rev 114:4175–4205
Acknowledgements
We would like to thank Anja Krieger-Liszkay (CEA Saclay) for discussions and technical suggestions for the trapping of ROS. This work was supported by JSPS-KAKENHI grant in Scientific Research on Innovative Areas “Innovations for Light-Energy Conversion (I4LEC)” (17H064351 for M. S.) and a JSPS-KAKENHI grant (17K07367 for M.S.). AB was supported in part by the French Infrastructure for Integrated Structural Biology (FRISBI) ANR-10-INBS-05.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, M., Boussac, A. & Sugiura, M. Consequences of structural modifications in cytochrome b559 on the electron acceptor side of Photosystem II. Photosynth Res 139, 475–486 (2019). https://doi.org/10.1007/s11120-018-0521-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-0521-0