Abstract
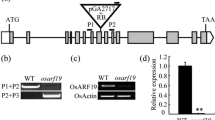
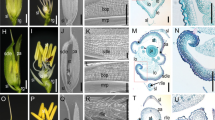
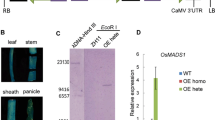
The development of the rice floral organ directly affects rice yield and quality, and rice spikelet development has always been the hotspot in the field of botanical research. Mutants of the rice floral organ are very important intermediate materials which are used to reveal the genetic mechanism of spikelet development. In the present study, a stable rice mutant named unclosed glumes (ucgl) with a Japonica background, of which the lemma and palea cannot close fully after flowering, was isolated from the population mutagenized by using the ethyl methane sulfonate (EMS) in rice. Results from the genetic analysis indicated that the mutant was controlled by a single recessive gene. The UCGL locus was mapped to a 118-kb region between SSR makers R8UM89 and RM23258 on chromosome 8. There was a C to T substitution at the fifth exon of LOC_Os08g0459600 by sequencing analysis. LOC_Os08g0459600 encodes 12-oxo-phytodienoic acid reductase 7 (OPR7), which is one of the key enzymes in the jasmonic acid (JA) biosynthesis. RT-PCR analysis showed that the UCGL gene could be expressed in all of the tested tissues including roots, stems, leaves, and panicles. Also, the concentration of endogenous JA in the ucgl mutant was reduced in contrast to the wild-type. The expression levels of JA biosynthetic genes including OsLOX and OsAOS were significantly increased in the ucgl mutant plant. The expression of UCGL (OsOPR7) could regulate JA metabolism and affect the closure of glumes in rice. As far as we know, the UCGL is thus a new gene and has not been reported as controlling the closure of rice glumes, thusthe study of it could provide a new insight into the role of JA in rice floral morphological development.








Similar content being viewed by others
Abbreviations
- Bp:
-
Base pair
- EMS:
-
Ethyl methanesulfonate
- JA:
-
Jasmonic acid
- InDel:
-
Insertion/deletion
- ORF:
-
Open reading frame
- SEM:
-
Scanning electron microscopy
- SSR:
-
Simple sequence repeat
- UTR:
-
Untranslated region
References
Angelo S, Marco T, Stefania DD, Stefania B, Palmiro P, Victoria P2, Victor F (2013) Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant Cell Rep 32:1085–1098
Bandara P, Karunagoda RP, Takahashi K, Nabeta K (2012) Cloning and functional characterization of key enzymes in putative octadecanoid pathway of physcomitrella patens. Trop Agric Res 23:160–167
Barazesh S, McSteen P (2008) Hormonal control of grass inflorescence development. Trends Plant Sci 13:12
Bolduc N, Hake S (2009) The maize transcription factor KNOTTED1 directly regulates the gibberellin catabolism gene ga2ox1. Plant Cell 21:1647–58
Breithaupt C, Kurzbauer R, Schaller F, Stintzi A, Schaller A, Huber R, Macheroux P, Clausen T (2009) Structural basis of substrate specificity of plant 12-oxophytodienoate reductases. J Mol Biol 392:1266–1277
Cai Q, Yuan Z, Chen M, Yin C, Luo Z, Zhao X, Liang W, Hu J, Zhang D (2014) Jasmonic acid regulates spikelet development in rice. Nat Commun 5:3476
Chehab EW, Kaspi R, Savchenko T, Rowe H, Negre-Zakharov F, Kliebenstein D, Dehesh K (2008) Distinct roles of jasmonates and aldehydes in plant-defense responses. Plos ONE 3:1904
Chen ZX, Wu JG, Ding WN, Chen HM, Wu P, Shi CH (2006) Morphogenesis and molecular basis on naked seed rice, a novel homeotic mutation of OsMADS1 regulating transcript level of AP3 homologue in rice. Planta 223:882–890
Cheng Y, Zhao Y (2007) A role for auxin in flower development. Plant Biol 49:99–104
Coen ES, Meyerowitz EM (1991) War of the whorls: genetic interactions controlling flower development. Nature 353:31–37
Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF (2004) The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol 14:1935–1940
Dreni L, Jacchia S, Fornara F, Fomari M, Ouwerkerk P, An G, Colombo L, Kater MM (2007) The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J 52(4):690–699
Agrawal GK, Tamogami S, Han O, Iwahashi H, Rakwal R (2004) Rice octadecanoid pathway. Biochem Bioph Res Co 317:1–15
Gao XQ, Zeng XC, Xia K, Yoshihara T, Zhou X (2004) Interactive effects of methyl jasmonate and salicylic on foret opening in spikelets of sorghum. Plant Growth Regul 43:269–273
He YM, Lin YJ, Zeng XC (2012) Dynamic changes of jasmonic acid biosynthesis in rice florets during natural anthesis. Acta Agron Sin (in Chinese with English abstract) 38:1891–1899
Ishiga Y, Funato A, Tachiki T, Toyoda K, Shiraishi T, Yamada T, Ichinose Y (2002) Expression of the 12-oxophytodienoic acid 10, 11-reductase gene in the compatible interaction between pea and fungal pathogen. Plant Cell Physiol 43:1210–1220
Jeon JS, Jang S, Lee S, Nam J, Kim C (2000) leafy hull sterile1 is a homeotic mutation in a rice MADS-Box gene affecting rice flower development. Plant Cell 12:871–884
Kalika P, Sriram P, Usha V (2005) OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs. Plant J 43:915–928
Kang HG, Noh YS, Chung YY, Costa M, An K, An G (1995) Phenotypic alterations of petals and sepals by ectopic expression of a rice MADS-box gene in tobacco. Plant Mol Biol 29:1–10
Kang HG, Jeon JS, Lee S, An G (1998) Identification of class B and class C floral organ identity genes from rice. Plant Mol Biol 38:1021–1029
Kyozuka J (2007) Control of shoot and root meristem function by cytokinin. Plant Biol 10:442–46
Li WY, Liu B, Yu LJ, Feng DR, Wang HB, Wang JF (2009) Phylogenetic analysis, structural evolution and functional divergence of the 12-oxo-phytodienoate acid reductase gene family in plants. BMC Evol Biol 9:90
Li HF, Liang WQ, Jia RD, Y'm CS, Zong J, Kong HZ, Zhang DB (2010a) The AGL6-like gene OsMADS6 regulates floral organ and meristem identities in rice OsMADS6 specifies flower development. Cell Res 20:299–313
Li WQ, Wu JG, Weng SL, Zhang YJ, Zhang DP, Shi CH (2010b) Identification and characterization of dwarf 62, a loss-of-function mutation in DLT/OsGRAS-32 affecting gibberellin metabolism in rice. Planta 232:1383–1396
Li H, Liang W, Yin C, Zhu L, Zhang D (2011a) Genetic interaction of OsMADS3, DROOPING LEAF, and OsMADS13 in specifying rice floral organ identities and meristem determinacy. Plant Physiol 156(1):263–274
Li WY, Zhou F, Liu B, Feng D, He YM, Qi KB, Wang HB, Wang JF (2011b) Comparative characterization, expression pattern and function analysis of the 12-oxo-phytodienoic acid reductase gene family in rice. Plant Cell Rep 30:981–995
Matsui H, Nakamura G, Ishiga Y, Toshima H, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y (2004) Structure and expression of 12-oxophytodienoate reductase (subgroup I) genes in pea, and characterization of the oxidoreductase activities of their recombinant products. Mol Genet Genomics 271:1–10
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nagasawa N, Miyoshi M, Sano Y, Satoh H, Hirano H, Sakai H, Nagato Y (2003) SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice. Development 130:705–718
Parthier B (1991) Jasmonates, new regulators of plant growth and development: many facts and few hypothesis on their actions. Bot Acta 104:405–464
Punita N, CM E, Hans W, SE P (2005) Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132:18
Riemann M, Riemann M, Takano M (2008) Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signaling. Plant Cell Environ 31:783–792
Schaller F, Weiler EW (1997) Molecular cloning and characterization of 12-oxophytodienoate reductase, an enzyme of the octadecanoid signaling pathway from Arabidopsis thaliana. J Biol Chem 272:28066–28072
Schaller F, Biesgen C, Mussig C, Altmann T, Weiler EW (2000) 12-Oxophytodienoic acid reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 210:979–984
Shinnosuke O, Mayumi K, Maiko S, Akio M, Hirohiko H, Eiji U, Yasuo N, Hitoshi Y (2009) Floral organs, an AGL6-Like MADS-Box gene, regulates floral organ identity and meristem fate in rice. Plant Cell 21:3008–3025
Staswick PE (1995) Jasmonates, salicylic acid and brassinolides. In: Davies PJ (ed) Plant hormones: physiology, biochemistry and molecular biology. Dordrecht, Netherlands pp 179–1873
Stintzi A, Browse J (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthsis. Proc Natl Acad Sci USA 97:10625–10630
Tani T, Sobajima H, Okada K, Chujo T, Arimura S, Tsutsumi N, Nishimura M, Seto H, Nojiri H, Yamane H (2008) Identification of the OsOPR7 gene encoding 12-oxophytodienoate reductase involved in the biosynthesis of jasmonic acid in rice. Planta 227:517–526
Theissen G, Saedler H (2001) Plant biology: floral quartets. Nature 409:469–471
Wang KJ, Tang D, Hong LI, Huang J, Li M, Gu MH, Xue YB, Cheng ZK (2010) DEP and AFO regulate reproductive habit in rice. Plos Genet 6:1
Wang SS, Wang CS, Tseng TH, Hou YL, Chen KY (2011) High-resolution genetic mapping and candidate gene indentification of the SLP1 locus that controls glume development in rice. Theor Appl Genet 122:1489–1496
Wang YM, Yan DW, Zhang YY, Li J, Cang J (2012) Regulation of floral organ identity in Arabidopsis by ectopic expression of OsMADS58. J Northeast Agric Univ 3 (English edition)
Wassim E, Chehab SK, Tatyana S, Daniel K, Katayoon D, Janet BI (2011) T-DNA insertion renders arabidopsis opr3 a conditional jasmonic acid-producing mutant. Plant Physiol 156:770–778
Weigel D, Meyerowitz EM (1994) The ABCs of floral homeotic genes. Cell 78:203–209
Yan ZF, Zhou X, Ma CH, Cui SP, Wei JK (2001) Inducing effect of coronatine and methyl jasmonate on the opening of spikelets in wheat, rye, mildew. Sci Agric Sin 34:334–337 (in Chinese with English abstract)
Yuan Z, Gao S, Xue DW, Luo D, Li LT, Ding SY, Yao X, Wilson ZA, Qian Q, Zhang DB (2009) Retarded palea1 controls palea development and floral zygomorphy in rice. Plant Physiol 149:235–244
Yun DP, Liang WQ, Drenic L, Yin CS, Zhou ZG, Kater MM, Zhang DB (2013) OsMADS16 Genetically Interacts with OsMADS3 and OsMADS58 in Specifying Floral Patterning in Rice. Mol Plant 6(3):743–756
Zeng SY (2010) Identifying and molecule mapping of glumes-unclosing genes in rice. Master thesis, Yangzhou University (in Chinese with English abstract)
Zeng XC, Zhou X, Zhang W, Murofushi N, Kitahara T, Kamuro Y (1999) Opening of rice floret in rapid response to methyl jasmonate. J Plant Growth Regul 18:153–158
Zhang DB, Yuan Z (2014) Molecular control of grass inflorescence development. Plant Biol 65:553–78
Zhang DB, Yuan Z, An G, Dreni LHJP, Kater M (2013) Panicle development. Genet Genomics Rice Plant Genet Genomics: Crops Models 5:279–295
Zhao Y (2008) The role of local biosynthesis of auxin and cytokinin in plant development. Plant Biol 11:16–22
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Plant Biol 61:49–64
Acknowledgements
This work was supported by National Science and Technology Support Program (2011BAD35B02), Zhejiang Provincial Natural Science Foundation of China (no. Z3100089), Science and Technology Office of Zhejiang Province (2012C12901-2), the Fundamental Research Funds for the Central Universities, and the Program for Innovative Research Team in University (IRT1185). We also thank Dr. Alfred Quampah and Dr. Rafaqat Ali Gill for revising the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, L., Shi, C.H., Zeng, D.D. et al. Morphogenesis and Molecular Basis on the Unclosed Glumes, a Novel Mutation Related to the Floral Organ of Rice. Plant Mol Biol Rep 33, 480–489 (2015). https://doi.org/10.1007/s11105-014-0764-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-014-0764-7